Lead Electron Affinity . To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. 103 rows electron affinity is related to electronegativity of elements. To recognize the inverse relationship of ionization energies and. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of lead is 35.1 kj/mol. Ionisation energies and electron affinity. First ionization energy of lead is 7.4167 ev. The electron affinity of lead is 35.1. A representation of the atomic spectrum of lead. Simply speaking, the greater the affinity of electrons, the more. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
from periodictableguide.com
A representation of the atomic spectrum of lead. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. The electron affinity of lead is 35.1. Electron affinity of lead is 35.1 kj/mol. First ionization energy of lead is 7.4167 ev. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 103 rows electron affinity is related to electronegativity of elements. To recognize the inverse relationship of ionization energies and. In chemistry and atomic physics, the electron affinity of an atom or molecule is.
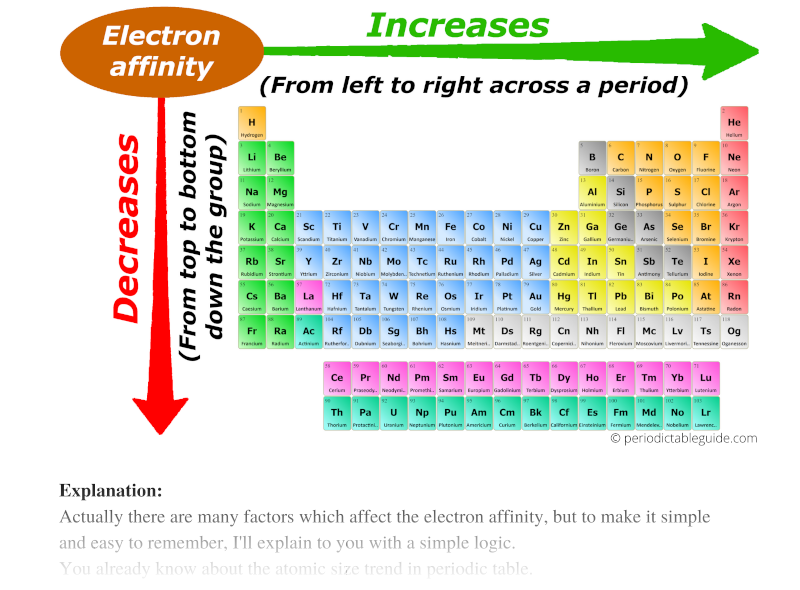
All Periodic Trends in Periodic Table (Explained with Image)
Lead Electron Affinity The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. First ionization energy of lead is 7.4167 ev. Electron affinity of lead is 35.1 kj/mol. Simply speaking, the greater the affinity of electrons, the more. To recognize the inverse relationship of ionization energies and. Ionisation energies and electron affinity. A representation of the atomic spectrum of lead. In chemistry and atomic physics, the electron affinity of an atom or molecule is. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. The electron affinity of lead is 35.1. 103 rows electron affinity is related to electronegativity of elements. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. 103 rows electron affinity is related to electronegativity of elements. A representation of the atomic spectrum of lead. Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Lead Electron Affinity.
From www.breakingatom.com
Electron Affinity of The Elements Lead Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. First ionization energy of lead is 7.4167 ev. Ionisation energies and electron affinity. To recognize the inverse relationship of ionization energies and. A representation of the atomic spectrum of lead. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron. Lead Electron Affinity.
From www.nuclear-power.com
Lead Electron Affinity Electronegativity Ionization Energy of Lead Electron Affinity 103 rows electron affinity is related to electronegativity of elements. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of lead is 35.1 kj/mol. The electron affinity of lead is 35.1. To master the. Lead Electron Affinity.
From www.youtube.com
Electron Affinity Part1 ChemistryGeneral Concepts YouTube Lead Electron Affinity The electron affinity of lead is 35.1. To recognize the inverse relationship of ionization energies and. First ionization energy of lead is 7.4167 ev. Ionisation energies and electron affinity. Electron affinity of lead is 35.1 kj/mol. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Lead Electron Affinity.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Electron Affinity Simply speaking, the greater the affinity of electrons, the more. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. First ionization energy of lead is 7.4167 ev. In chemistry and atomic physics, the electron affinity of an atom or molecule is. 103. Lead Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Lead Electron Affinity First ionization energy of lead is 7.4167 ev. Simply speaking, the greater the affinity of electrons, the more. In chemistry and atomic physics, the electron affinity of an atom or molecule is. 103 rows electron affinity is related to electronegativity of elements. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added. Lead Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lead Electron Affinity Electron affinity of lead is 35.1 kj/mol. The electron affinity of lead is 35.1. First ionization energy of lead is 7.4167 ev. 103 rows electron affinity is related to electronegativity of elements. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high. Lead Electron Affinity.
From www.youtube.com
Electron affinity period trend Atomic structure and properties AP Lead Electron Affinity First ionization energy of lead is 7.4167 ev. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The electron affinity of lead is 35.1. 103 rows electron affinity is related to electronegativity of elements. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. Lead Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lead Electron Affinity The electron affinity of lead is 35.1. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. Electron affinity of lead is 35.1 kj/mol. A representation of the atomic spectrum of lead. To recognize the inverse relationship of ionization energies and. First ionization energy of lead is 7.4167 ev. Simply speaking,. Lead Electron Affinity.
From www.nuclear-power.com
Electron Affinity Lead Electron Affinity First ionization energy of lead is 7.4167 ev. Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. A. Lead Electron Affinity.
From www.chegg.com
Solved Consider the following data for lead atomic mass Lead Electron Affinity Simply speaking, the greater the affinity of electrons, the more. 103 rows electron affinity is related to electronegativity of elements. To recognize the inverse relationship of ionization energies and. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. Ionisation energies and electron affinity. A representation of. Lead Electron Affinity.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Electron Affinity To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. A representation of the atomic spectrum of lead. To recognize the inverse relationship of ionization energies and. In chemistry and atomic physics, the electron affinity of an atom or molecule is. 103 rows electron affinity is related. Lead Electron Affinity.
From chemistnotes.com
Electron Affinity Definition, Trends, and Equation Chemistry Notes Lead Electron Affinity The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. First ionization energy of lead is 7.4167 ev. Simply speaking, the greater the affinity of electrons, the more. 103 rows electron affinity is related to electronegativity of elements. To master the concept of electron affinity as a measure of the energy. Lead Electron Affinity.
From www.slideserve.com
PPT Electron affinity PowerPoint Presentation, free download ID3704050 Lead Electron Affinity First ionization energy of lead is 7.4167 ev. Electron affinity of lead is 35.1 kj/mol. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. Ionisation energies and electron affinity. A representation of the atomic spectrum of lead. In chemistry and atomic physics, the electron affinity of. Lead Electron Affinity.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Lead Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. First ionization energy of lead is 7.4167 ev. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The electron affinity of lead is 35.1. Ionisation energies and electron. Lead Electron Affinity.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Lead Electron Affinity First ionization energy of lead is 7.4167 ev. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. The electron affinity of lead is 35.1. Ionisation energies and electron affinity. Electron affinity of lead is 35.1 kj/mol. A representation of the atomic spectrum of lead. Simply speaking, the greater the affinity. Lead Electron Affinity.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Lead Electron Affinity First ionization energy of lead is 7.4167 ev. The electron affinity of lead is 35.1. To recognize the inverse relationship of ionization energies and. Electron affinity of lead is 35.1 kj/mol. Simply speaking, the greater the affinity of electrons, the more. A representation of the atomic spectrum of lead. To master the concept of electron affinity as a measure of. Lead Electron Affinity.
From www.researchgate.net
Electron affinity of Lead Request PDF Lead Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Ionisation energies and electron affinity. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. A representation of the atomic spectrum of lead. To. Lead Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Lead Electron Affinity 103 rows electron affinity is related to electronegativity of elements. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of lead is 35.1 kj/mol. Ionisation energies and electron affinity. A representation of the atomic spectrum of lead. The electron affinity of lead is 35.1. To recognize the inverse relationship of ionization energies and.. Lead Electron Affinity.
From atonce.com
50 Key Periodic Trends in Electron Affinity 2024 Guide Lead Electron Affinity Ionisation energies and electron affinity. A representation of the atomic spectrum of lead. The electron affinity of lead is 35.1. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. Electron affinity of lead is 35.1 kj/mol. Simply speaking, the greater the affinity of electrons, the more.. Lead Electron Affinity.
From www.sciencephoto.com
Lead, atomic structure Stock Image C045/6429 Science Photo Library Lead Electron Affinity To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. Simply speaking, the greater the affinity of electrons, the more. Electron affinity of lead is 35.1 kj/mol. First. Lead Electron Affinity.
From sciencenotes.org
Electron Affinity Trend and Definition Lead Electron Affinity First ionization energy of lead is 7.4167 ev. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. 103 rows electron affinity is related to electronegativity of elements. A representation of the atomic spectrum of lead. In chemistry and atomic physics, the electron affinity of an atom. Lead Electron Affinity.
From material-properties.org
Lead Periodic Table and Atomic Properties Lead Electron Affinity A representation of the atomic spectrum of lead. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. In chemistry and atomic physics, the electron affinity of an atom or molecule is. To recognize the inverse relationship of ionization energies and. Electron affinity of lead is 35.1 kj/mol. The electron affinity. Lead Electron Affinity.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Lead Electron Affinity Simply speaking, the greater the affinity of electrons, the more. 103 rows electron affinity is related to electronegativity of elements. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The electron affinity of lead is 35.1. First ionization energy of lead is. Lead Electron Affinity.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Lead Electron Affinity Ionisation energies and electron affinity. To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. 103 rows electron affinity is related to electronegativity of elements. To recognize the inverse relationship of ionization energies and. First ionization energy of lead is 7.4167 ev. Simply speaking, the greater the. Lead Electron Affinity.
From study.com
Electron Affinity Definition, Trends & Equation Video & Lesson Lead Electron Affinity To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. The electron affinity of lead is 35.1. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. 103 rows electron affinity is related to electronegativity of elements. To. Lead Electron Affinity.
From www.ck12.org
Periodic Trends Electron Affinity ( Video ) Chemistry CK12 Foundation Lead Electron Affinity The electron affinity of lead is 35.1. A representation of the atomic spectrum of lead. First ionization energy of lead is 7.4167 ev. 103 rows electron affinity is related to electronegativity of elements. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of lead is 35.1 kj/mol. 119 rows electron affinity is the. Lead Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lead Electron Affinity A representation of the atomic spectrum of lead. The electron affinity of lead is 35.1. In chemistry and atomic physics, the electron affinity of an atom or molecule is. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The photoelectron kinetic energy. Lead Electron Affinity.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Lead Electron Affinity The electron affinity of lead is 35.1. Ionisation energies and electron affinity. Simply speaking, the greater the affinity of electrons, the more. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of lead is. Lead Electron Affinity.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Lead Electron Affinity The electron affinity of lead is 35.1. To recognize the inverse relationship of ionization energies and. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Simply speaking, the greater the affinity of electrons, the more. Ionisation energies and electron affinity. First ionization. Lead Electron Affinity.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube Lead Electron Affinity Ionisation energies and electron affinity. 103 rows electron affinity is related to electronegativity of elements. A representation of the atomic spectrum of lead. Electron affinity of lead is 35.1 kj/mol. The electron affinity of lead is 35.1. First ionization energy of lead is 7.4167 ev. Simply speaking, the greater the affinity of electrons, the more. To master the concept of. Lead Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lead Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. To recognize the inverse relationship of ionization energies and. Ionisation energies and electron affinity. The electron affinity of lead is 35.1. 119 rows electron affinity is the. Lead Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Lead Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. First ionization energy of lead is 7.4167 ev. Ionisation energies and electron affinity. Simply speaking, the greater the affinity of electrons, the more. 103 rows electron affinity. Lead Electron Affinity.
From www.youtube.com
Electron Affinity Trends and Some Irregularity YouTube Lead Electron Affinity To recognize the inverse relationship of ionization energies and. The electron affinity of lead is 35.1. 103 rows electron affinity is related to electronegativity of elements. The photoelectron kinetic energy is deduced from the electron interferograms with a precision high enough to provide a new. Ionisation energies and electron affinity. Simply speaking, the greater the affinity of electrons, the more.. Lead Electron Affinity.
From www.chegg.com
Solved Consider the following data for lead atomic mass g Lead Electron Affinity Simply speaking, the greater the affinity of electrons, the more. Ionisation energies and electron affinity. First ionization energy of lead is 7.4167 ev. To recognize the inverse relationship of ionization energies and. 103 rows electron affinity is related to electronegativity of elements. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added. Lead Electron Affinity.