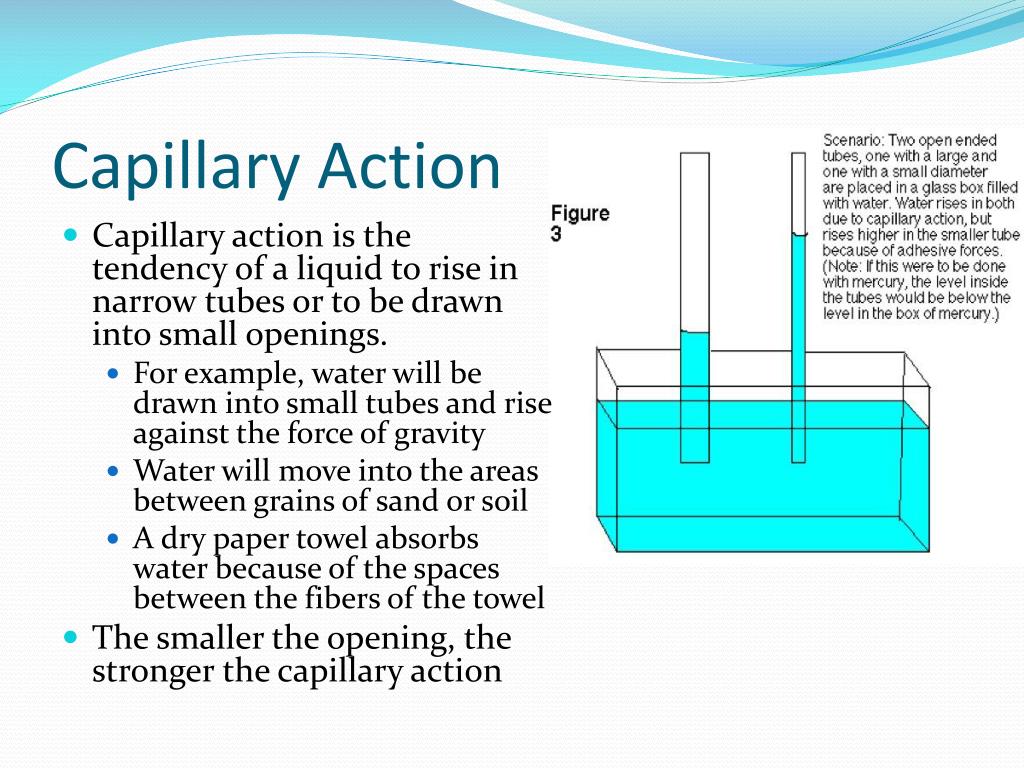
Surface Tension And Capillary Action Occur In Water Because It . Capillary action occurs when the adhesion to the walls is stronger than the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. The surface tension acts to hold the surface intact. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside.
from www.slideserve.com
Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension acts to hold the surface intact. The surface tension is measured in. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
PPT Horticultural Responses to Water PowerPoint Presentation, free
Surface Tension And Capillary Action Occur In Water Because It Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension is measured in. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension acts to hold the surface intact.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Surface Tension And Capillary Action Occur In Water Because It The surface tension is measured in. Capillary action occurs when the adhesion to the walls is stronger than the. Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension acts to. Surface Tension And Capillary Action Occur In Water Because It.
From www.yourhop.com
How [Surface Tension] and [Capillary Action] Work in Water / Soil With Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Surface tension is the reason why liquids form bubbles and droplets. Capillary action occurs when the adhesion to the walls is stronger than the. Cohesive forces between molecules cause the surface of a liquid to. Surface Tension And Capillary Action Occur In Water Because It.
From anabelximorales.blogspot.com
Which Best Explains the Surface Tension of Water Capillary Action Surface Tension And Capillary Action Occur In Water Because It Surface tension is the reason why liquids form bubbles and droplets. The surface tension acts to hold the surface intact. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest. Surface Tension And Capillary Action Occur In Water Because It.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Surface Tension And Capillary Action Occur In Water Because It The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the. Surface tension is the reason why liquids form bubbles and droplets. The surface tension is measured in. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideserve.com
PPT Horticultural Responses to Water PowerPoint Presentation, free Surface Tension And Capillary Action Occur In Water Because It Capillary action occurs when the adhesion to the walls is stronger than the. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension acts to hold the surface intact. Cohesive forces between molecules cause the surface of a liquid to contract to. Surface Tension And Capillary Action Occur In Water Because It.
From www.numerade.com
At 60^∘ C the surface tension of mercury and water is 0.47 and 0.0662 N Surface Tension And Capillary Action Occur In Water Because It It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. Capillary action occurs when the adhesion to the walls is stronger than the. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface. Surface Tension And Capillary Action Occur In Water Because It.
From www.researchgate.net
A schematic of water capillary imbibition. Download Scientific Diagram Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Surface tension is the reason why. Surface Tension And Capillary Action Occur In Water Because It.
From www.tutorix.com
To find the Surface Tension of Water by Capillary Rise Method Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. It can be shown that the. Surface Tension And Capillary Action Occur In Water Because It.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Surface Tension And Capillary Action Occur In Water Because It It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. This occurs as a result of like molecules, cohesive forces, banding together to form a. Surface Tension And Capillary Action Occur In Water Because It.
From tikz.net
Surface tension & capillary action Surface Tension And Capillary Action Occur In Water Because It Capillary action occurs when the adhesion to the walls is stronger than the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Surface tension is the. Surface Tension And Capillary Action Occur In Water Because It.
From sciencenotes.org
Capillary Action What It Is and How It Works Surface Tension And Capillary Action Occur In Water Because It It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. Capillary action occurs when the adhesion to the walls is stronger than the. Surface tension is the reason why liquids form bubbles and droplets. The surface tension acts to hold the surface intact. This occurs as a result of like. Surface Tension And Capillary Action Occur In Water Because It.
From ar.inspiredpencil.com
Water Capillary Action Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Cohesive forces between molecules cause the surface of a liquid to contract. Surface Tension And Capillary Action Occur In Water Because It.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action Surface Tension And Capillary Action Occur In Water Because It Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension acts to hold the surface intact. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the. Surface Tension And Capillary Action Occur In Water Because It.
From www.studypool.com
SOLUTION Physics 101 surface tension and capillary effect and Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. It. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… Surface Tension And Capillary Action Occur In Water Because It Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension is measured in. The surface tension acts to hold the surface intact. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. It can be shown that the gauge. Surface Tension And Capillary Action Occur In Water Because It.
From www.yourhop.com
How [Surface Tension] and [Capillary Action] Work in Water / Soil With Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension is measured in. Capillary action occurs when the adhesion. Surface Tension And Capillary Action Occur In Water Because It.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Surface Tension And Capillary Action Occur In Water Because It Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension acts to hold the surface intact. The surface tension is measured in. Cohesive forces between molecules cause the surface of a. Surface Tension And Capillary Action Occur In Water Because It.
From www.vedantu.com
Differentiate between Hygroscopic water and capillary water. Surface Tension And Capillary Action Occur In Water Because It Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by. Surface Tension And Capillary Action Occur In Water Because It.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension And Capillary Action Occur In Water Because It It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. The surface tension is measured in. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of. Surface Tension And Capillary Action Occur In Water Because It.
From www.yourhop.com
How [Surface Tension] and [Capillary Action] Work in Water / Soil With Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Capillary action occurs when the adhesion to the walls is stronger than the. Surface tension is the reason why liquids form bubbles and droplets. It can be shown that the gauge pressure \(p \) inside. Surface Tension And Capillary Action Occur In Water Because It.
From www.ddcoatings.co.uk
What is Capillary Action? Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension acts to hold the surface intact. Capillary action occurs. Surface Tension And Capillary Action Occur In Water Because It.
From www.coursehero.com
[Solved] how will you explain surface tension and capillarity to an Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Surface tension is the reason why liquids form bubbles and droplets. Capillary action occurs when the adhesion to the walls is stronger than the. It can be shown that the gauge pressure \(p \) inside. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideserve.com
PPT Plant Transport of Water and Nutrients PowerPoint Presentation Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion. Surface Tension And Capillary Action Occur In Water Because It.
From www.youtube.com
Surface tension and capillary rise YouTube Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Capillary action occurs when the adhesion to the walls is stronger than the. The inward surface tension force causes. Surface Tension And Capillary Action Occur In Water Because It.
From slideplayer.com
Water. ppt download Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension acts to hold the surface intact. Surface tension is the reason why liquids form bubbles and droplets. The surface tension is measured in. Cohesive forces between molecules cause the surface of a. Surface Tension And Capillary Action Occur In Water Because It.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension And Capillary Action Occur In Water Because It The surface tension acts to hold the surface intact. The surface tension is measured in. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Capillary action. Surface Tension And Capillary Action Occur In Water Because It.
From www.youtube.com
Chemistry Explained Viscosity, Surface Tension, Adhesion, Cohesion Surface Tension And Capillary Action Occur In Water Because It It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension acts to hold the surface intact. This occurs as a result of like molecules, cohesive forces, banding together to. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideserve.com
PPT Human Biology (BIOL 104) PowerPoint Presentation, free download Surface Tension And Capillary Action Occur In Water Because It The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. The surface tension acts to hold the surface intact. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. This occurs as a result of. Surface Tension And Capillary Action Occur In Water Because It.
From study.com
Capillary Action of Water Definition & Examples Video & Lesson Surface Tension And Capillary Action Occur In Water Because It Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension acts to hold the surface intact. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension is measured in. The inward surface tension force causes bubbles to be approximately spherical and raises the. Surface Tension And Capillary Action Occur In Water Because It.
From tikz.net
Surface tension & capillary action Surface Tension And Capillary Action Occur In Water Because It The surface tension acts to hold the surface intact. This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Capillary action occurs. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideserve.com
PPT Plant Physiology 2014 PowerPoint Presentation, free download ID Surface Tension And Capillary Action Occur In Water Because It Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. Capillary action occurs when the adhesion to the walls is stronger than the. The surface tension is. Surface Tension And Capillary Action Occur In Water Because It.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped. Surface Tension And Capillary Action Occur In Water Because It.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts Surface Tension And Capillary Action Occur In Water Because It The surface tension is measured in. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Capillary action occurs when the adhesion to the walls is stronger than the. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric. Surface Tension And Capillary Action Occur In Water Because It.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Surface Tension And Capillary Action Occur In Water Because It This occurs as a result of like molecules, cohesive forces, banding together to form a somewhat impenetrable surface on the body of water. The surface tension is measured in. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. The surface tension acts to hold the surface intact. The inward. Surface Tension And Capillary Action Occur In Water Because It.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download Surface Tension And Capillary Action Occur In Water Because It The surface tension acts to hold the surface intact. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension is measured in. It can be shown that the gauge pressure \(p \) inside a spherical bubble is given by \[p = \dfrac{4\gamma. Surface tension is the reason why liquids. Surface Tension And Capillary Action Occur In Water Because It.