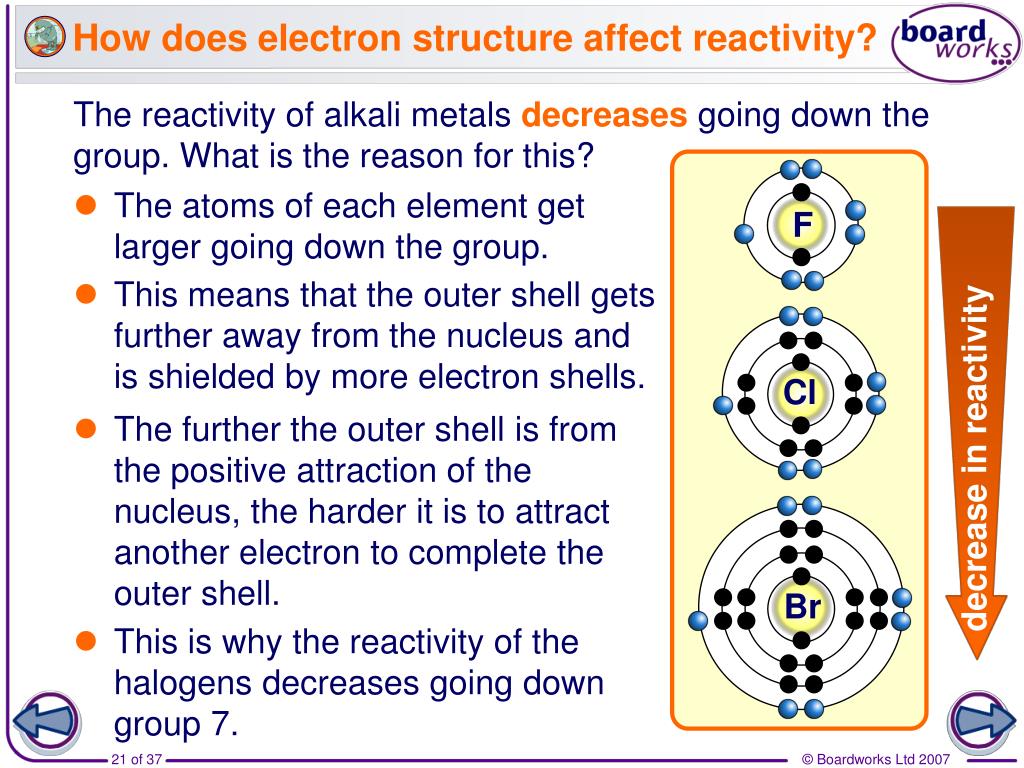
Why Is Chlorine In Group 7 . The word ‘halogen’ means 'salt former'. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The elements in group 7 are called the. The three common group 7 elements are chlorine, bromine and iodine. The group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. This is a disproportionation reaction. The fall in reactivity is because the halogens become less good oxidising agents as you go. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The group 7 elements are also known as the halogens. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide.
from www.slideserve.com
The word ‘halogen’ means 'salt former'. The three common group 7 elements are chlorine, bromine and iodine. The group 7 elements are also known as the halogens. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The elements in group 7 are called the. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The group 7 elements are also known as the halogens. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The fall in reactivity is because the halogens become less good oxidising agents as you go. This is a disproportionation reaction.
PPT Group 7 the halogens PowerPoint Presentation, free download
Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The elements in group 7 are called the. This is a disproportionation reaction. The fall in reactivity is because the halogens become less good oxidising agents as you go. The word ‘halogen’ means 'salt former'. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. The group 7 elements are also known as the halogens. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. At room temperature (20 °c), the physical state of the halogens changes as you go down the group.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. The word ‘halogen’ means 'salt former'. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common group 7 elements are chlorine, bromine and iodine. This is a disproportionation reaction. The group 7 elements are also known as the halogens. The fall in. Why Is Chlorine In Group 7.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The three common group 7 elements are chlorine, bromine and iodine. This is a disproportionation reaction. The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens.. Why Is Chlorine In Group 7.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Why Is Chlorine In Group 7 This is a disproportionation reaction. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens. Elements in the same. Why Is Chlorine In Group 7.
From www.britannica.com
Chlorine Uses, Properties, & Facts Britannica Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The word ‘halogen’ means 'salt former'. The three common group 7 elements are chlorine, bromine and iodine. Elements in the same group of the periodic table show trends in physical properties, such as boiling. Why Is Chlorine In Group 7.
From elchoroukhost.net
Chlorine Periodic Table Elcho Table Why Is Chlorine In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. This is a disproportionation reaction. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The elements in group 7 are called the. Elements in the same group of the periodic table show trends in physical properties, such as boiling point.. Why Is Chlorine In Group 7.
From inutoneko.info
Chlorine Uses Why Is Chlorine In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. At room temperature (20 °c), the physical state of the halogens changes as you. Why Is Chlorine In Group 7.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The fall in reactivity is because the halogens become less good oxidising agents as you go. The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens.. Why Is Chlorine In Group 7.
From studylib.net
Group 7 Elements Why Is Chlorine In Group 7 Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The group 7 elements are also known as the halogens. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common group 7 elements are chlorine, bromine and iodine. The group 7 elements are also. Why Is Chlorine In Group 7.
From sciencenotes.org
Element Families on the Periodic Table Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. This is a disproportionation reaction. The word ‘halogen’ means 'salt former'. The elements in group 7 are called the. The. Why Is Chlorine In Group 7.
From chem.libretexts.org
10.3 Compounds of Chlorine Chemistry LibreTexts Why Is Chlorine In Group 7 The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens. The fall in reactivity is because the halogens become less good oxidising agents as you go. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. This is a disproportionation reaction. Elements in the same group. Why Is Chlorine In Group 7.
From revisechemistry.uk
Groups in the Periodic Table Edexcel T6 revisechemistry.uk Why Is Chlorine In Group 7 At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. An element placed in group 7 of. Why Is Chlorine In Group 7.
From cabinet.matttroy.net
Chlorine Periodic Table Group Matttroy Why Is Chlorine In Group 7 Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. This is a disproportionation reaction. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The group 7 elements are also known as the halogens. The fall in reactivity is because the halogens become less good oxidising agents. Why Is Chlorine In Group 7.
From www.dreamstime.com
Chlorine Periodic Table of the Elements Vector Stock Vector Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. The elements in group 7 are called the. The group 7 elements are also known as the halogens. This is a disproportionation reaction. Elements in the same group. Why Is Chlorine In Group 7.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. The word ‘halogen’ means 'salt former'. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. An element placed in group 7 of the periodic table, which starts with fluorine. Why Is Chlorine In Group 7.
From periodictableguide.com
Chlorine (Cl) Periodic Table (Element Information & More) Why Is Chlorine In Group 7 This is a disproportionation reaction. The group 7 elements are also known as the halogens. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide.. Why Is Chlorine In Group 7.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Why Is Chlorine In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The three common group 7 elements are chlorine, bromine and iodine. The elements in group 7 are called the. The three common group 7 elements are chlorine, bromine and iodine. The group 7 elements are also known as the halogens. Chlorine and bromine produce iron (iii). Why Is Chlorine In Group 7.
From studymind.co.uk
Group 7 (GCSE Chemistry) Study Mind Why Is Chlorine In Group 7 An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The group 7 elements are also known as the halogens. This is a disproportionation reaction. The three common group 7 elements are chlorine, bromine and iodine. The fall in reactivity is because the halogens become less good oxidising agents as you go. The. Why Is Chlorine In Group 7.
From www.youtube.com
Chlorine Electron Configuration YouTube Why Is Chlorine In Group 7 Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common group 7 elements are chlorine, bromine and iodine.. Why Is Chlorine In Group 7.
From periodictable.me
How To Find The Electron Configuration For Chlorine Why Is Chlorine In Group 7 This is a disproportionation reaction. The fall in reactivity is because the halogens become less good oxidising agents as you go. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The three common group 7 elements are chlorine, bromine and iodine. The elements in group 7 are called the. The three common. Why Is Chlorine In Group 7.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The group 7 elements are also known as the halogens. The elements in group 7 are called the. Elements in the same group of the periodic table show trends in physical properties, such as boiling. Why Is Chlorine In Group 7.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Why Is Chlorine In Group 7 At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The three common group 7 elements are chlorine, bromine and iodine. The three common group 7 elements are chlorine, bromine and iodine. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. Fluorine and chlorine. Why Is Chlorine In Group 7.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Why Is Chlorine In Group 7 The elements in group 7 are called the. This is a disproportionation reaction. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. An element placed in group 7 of the periodic table, which starts with fluorine. Why Is Chlorine In Group 7.
From www.thesciencehive.co.uk
Group 7 (Halogens) (GCSE) — the science sauce Why Is Chlorine In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The fall in reactivity is because the halogens become less good oxidising agents as you go. Elements in the same group of the periodic table show trends in physical. Why Is Chlorine In Group 7.
From chemisfast.blogspot.com
Why is chlorine, ortho and para directing and Why ‘Cl’atom can not Why Is Chlorine In Group 7 The word ‘halogen’ means 'salt former'. This is a disproportionation reaction. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The elements in group 7 are called the. The group 7 elements are also known as the halogens. The group. Why Is Chlorine In Group 7.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Why Is Chlorine In Group 7 Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The three common group 7 elements are chlorine, bromine and iodine. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common group 7 elements are chlorine, bromine and iodine. The word ‘halogen’ means 'salt. Why Is Chlorine In Group 7.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Why Is Chlorine In Group 7 The word ‘halogen’ means 'salt former'. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The three common. Why Is Chlorine In Group 7.
From www.elevise.co.uk
C1 M) Group 1 Elements AQA Combined Science Trilogy Elevise Why Is Chlorine In Group 7 At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The elements in group 7 are called the. The group 7 elements are also known as the halogens. The word ‘halogen’ means 'salt former'. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common. Why Is Chlorine In Group 7.
From stock.adobe.com
Chlorine big on periodic Table of the Elements with atomic number Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. The group 7 elements are also known as the halogens. The word ‘halogen’ means 'salt former'. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. Elements in the same group of the periodic table show trends in physical properties, such as boiling. Why Is Chlorine In Group 7.
From chemicalengineeringworld.com
Chlorine in periodic table Archives Chemical Engineering World Why Is Chlorine In Group 7 The three common group 7 elements are chlorine, bromine and iodine. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. This is a disproportionation reaction. The fall in reactivity is because the halogens become less good oxidising agents as you go. The word ‘halogen’ means 'salt former'. The elements in group. Why Is Chlorine In Group 7.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Why Is Chlorine In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The fall in reactivity is because the halogens become less good oxidising agents as you go. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The group 7 elements are also known as the halogens. The three common. Why Is Chlorine In Group 7.
From www.onlinechemistrytutor.net
chlorine001 Online Chemistry Tutor Why Is Chlorine In Group 7 Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The three common group 7 elements are chlorine, bromine and iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. An element placed in group 7 of the periodic table, which starts with fluorine and ends with.. Why Is Chlorine In Group 7.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Why Is Chlorine In Group 7 The word ‘halogen’ means 'salt former'. The group 7 elements are also known as the halogens. The three common group 7 elements are chlorine, bromine and iodine. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The three common group 7 elements are chlorine, bromine and iodine. Elements in the same group. Why Is Chlorine In Group 7.
From www.theowletscience.org
Chemistry The Periodic Table of the Elements. Chlorine the owlet Why Is Chlorine In Group 7 The elements in group 7 are called the. An element placed in group 7 of the periodic table, which starts with fluorine and ends with. Elements in the same group of the periodic table show trends in physical properties, such as boiling point. The fall in reactivity is because the halogens become less good oxidising agents as you go. Fluorine. Why Is Chlorine In Group 7.
From www.animalia-life.club
Chlorine Element Periodic Table Why Is Chlorine In Group 7 An element placed in group 7 of the periodic table, which starts with fluorine and ends with. The fall in reactivity is because the halogens become less good oxidising agents as you go. The word ‘halogen’ means 'salt former'. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The three common group 7 elements are. Why Is Chlorine In Group 7.
From www.slideserve.com
PPT Chlorine PowerPoint Presentation, free download ID2277012 Why Is Chlorine In Group 7 The group 7 elements are also known as the halogens. Chlorine and bromine produce iron (iii) chloride or bromide, but iodine produces iron (ii) iodide. The three common group 7 elements are chlorine, bromine and iodine. The fall in reactivity is because the halogens become less good oxidising agents as you go. An element placed in group 7 of the. Why Is Chlorine In Group 7.