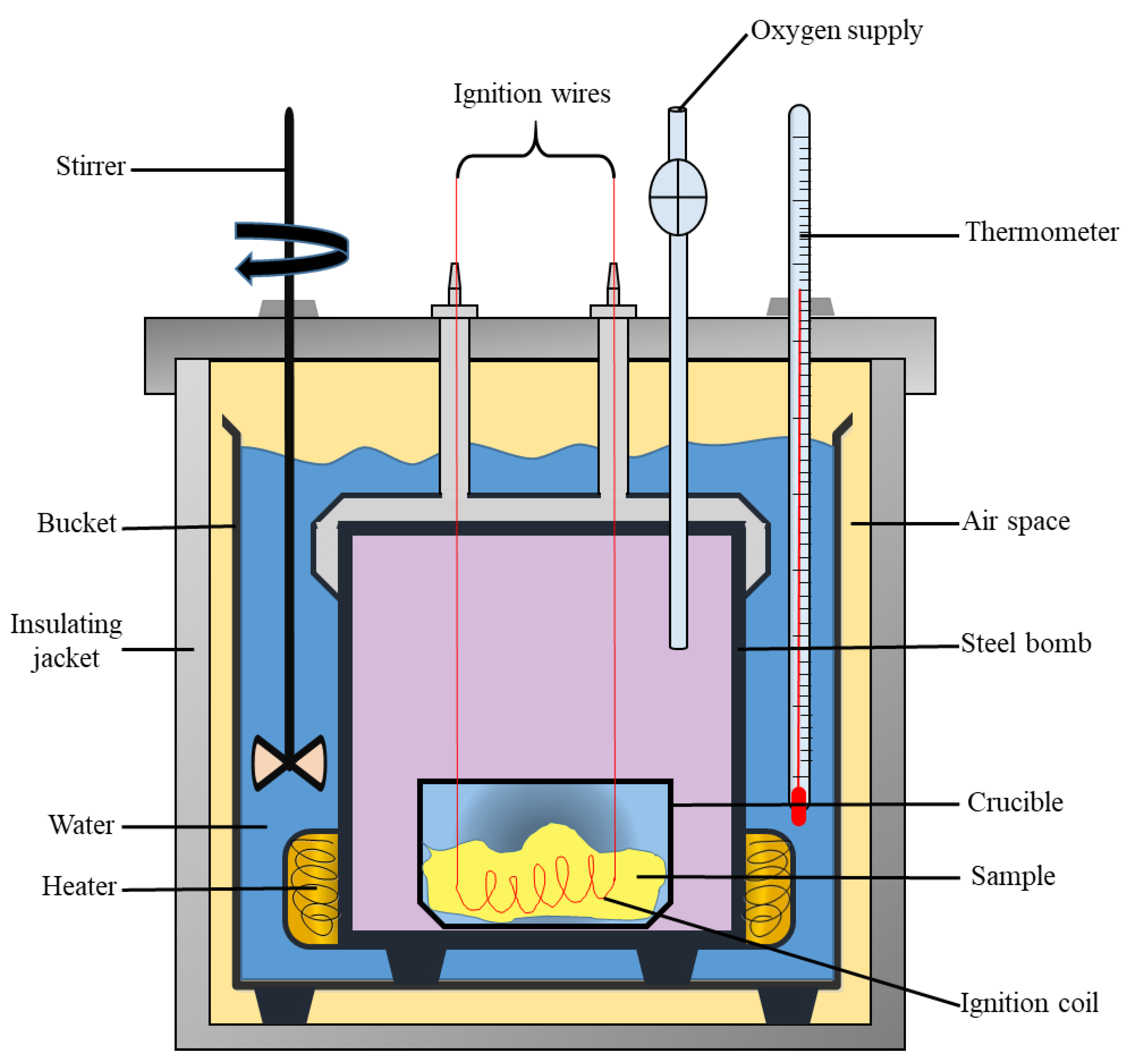
Calorimetry Calorimeter Chemistry . one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. Calorimetry is used to measure. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. apply the first law of thermodynamics to calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the.
from www.animalia-life.club
one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. From a chemical reaction like. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure.
Calorimeter Diagram
Calorimetry Calorimeter Chemistry Calorimetry is used to measure. From a chemical reaction like. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Calorimetry is used to measure. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Calorimeter Chemistry in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure. chemists use calorimetry to determine. Calorimetry Calorimeter Chemistry.
From hxeenedvw.blob.core.windows.net
Calorimeter Chemistry Ia at Beverly McPhee blog Calorimetry Calorimeter Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimetry Calorimeter Chemistry.
From users.highland.edu
Calorimetry Calorimetry Calorimeter Chemistry apply the first law of thermodynamics to calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure. Calorimetry Calorimeter Chemistry.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Calorimeter Chemistry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Calorimetry Calorimeter Chemistry.
From hxexppvfq.blob.core.windows.net
Ap Chemistry Calorimetry Problems at Christopher Redmond blog Calorimetry Calorimeter Chemistry From a chemical reaction like. Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter. Calorimetry Calorimeter Chemistry.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimetry Calorimeter Chemistry chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Compare. Calorimetry Calorimeter Chemistry.
From lessonfullsclerites.z13.web.core.windows.net
Coffee Cup Calorimetry Equation Calorimetry Calorimeter Chemistry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry. Calorimetry Calorimeter Chemistry.
From hxehitqky.blob.core.windows.net
Calorimetry Steps at Nicholas Larsen blog Calorimetry Calorimeter Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. From a chemical reaction like. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. one technique we can use to measure the amount of heat involved in a chemical. Calorimetry Calorimeter Chemistry.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Calorimeter Chemistry From a chemical reaction like. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is. Calorimetry Calorimeter Chemistry.
From www.animalia-life.club
Food Calorimeter Calorimetry Calorimeter Chemistry From a chemical reaction like. apply the first law of thermodynamics to calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure. . Calorimetry Calorimeter Chemistry.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Calorimetry Calorimeter Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. . Calorimetry Calorimeter Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Calorimeter Chemistry apply the first law of thermodynamics to calorimetry. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold. Calorimetry Calorimeter Chemistry.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimetry Calorimeter Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of. Calorimetry Calorimeter Chemistry.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Calorimeter Chemistry a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. From a chemical reaction like. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached. Calorimetry Calorimeter Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Calorimeter Chemistry chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Calorimetry is used to measure. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. apply the first law of thermodynamics to calorimetry. one technique we can use to measure the. Calorimetry Calorimeter Chemistry.
From saylordotorg.github.io
Calorimetry Calorimetry Calorimeter Chemistry Calorimetry is used to measure. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. one technique we can use to. Calorimetry Calorimeter Chemistry.
From hxexppvfq.blob.core.windows.net
Ap Chemistry Calorimetry Problems at Christopher Redmond blog Calorimetry Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is used to measure. . Calorimetry Calorimeter Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Calorimeter Chemistry calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. in. Calorimetry Calorimeter Chemistry.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Calorimeter Chemistry From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. one technique we can use to measure the amount of heat involved in a chemical or physical. Calorimetry Calorimeter Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Calorimeter Chemistry From a chemical reaction like. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure. chemists use calorimetry to determine the amount of heat transferred to or from a system into its. Calorimetry Calorimeter Chemistry.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimetry Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Compare heat flow from hot. Calorimetry Calorimeter Chemistry.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Calorimeter Chemistry chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is. Calorimetry Calorimeter Chemistry.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Calorimeter Chemistry calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Calorimeter Chemistry.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Calorimeter Chemistry Calorimetry is used to measure. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. apply the first law of thermodynamics to calorimetry. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. one technique we. Calorimetry Calorimeter Chemistry.
From lessonfullsclerites.z13.web.core.windows.net
Coffee Cup Calorimeter Formula Calorimetry Calorimeter Chemistry From a chemical reaction like. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances. Calorimetry Calorimeter Chemistry.
From exooqotfj.blob.core.windows.net
Calorimeter Chemistry at Roberta Burgett blog Calorimetry Calorimeter Chemistry chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. apply the first law of thermodynamics to calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor. Calorimetry Calorimeter Chemistry.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. . Calorimetry Calorimeter Chemistry.
From hxexppvfq.blob.core.windows.net
Ap Chemistry Calorimetry Problems at Christopher Redmond blog Calorimetry Calorimeter Chemistry calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is used to measure. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. in chemistry and thermodynamics, calorimetry (from latin. Calorimetry Calorimeter Chemistry.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimetry Calorimeter Chemistry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the.. Calorimetry Calorimeter Chemistry.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Calorimeter Chemistry Calorimetry is used to measure. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. one technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimetry Calorimeter Chemistry.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Calorimeter Chemistry From a chemical reaction like. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. apply the first law of thermodynamics to calorimetry. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. a calorimeter is a device used to measure. Calorimetry Calorimeter Chemistry.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter To Predict A Curve Calorimetry Calorimeter Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is. Calorimetry Calorimeter Chemistry.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimetry Calorimeter Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. From a chemical reaction like. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Calorimeter Chemistry.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Calorimeter Chemistry chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter. Calorimetry Calorimeter Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Calorimeter Chemistry one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a. Calorimetry Calorimeter Chemistry.