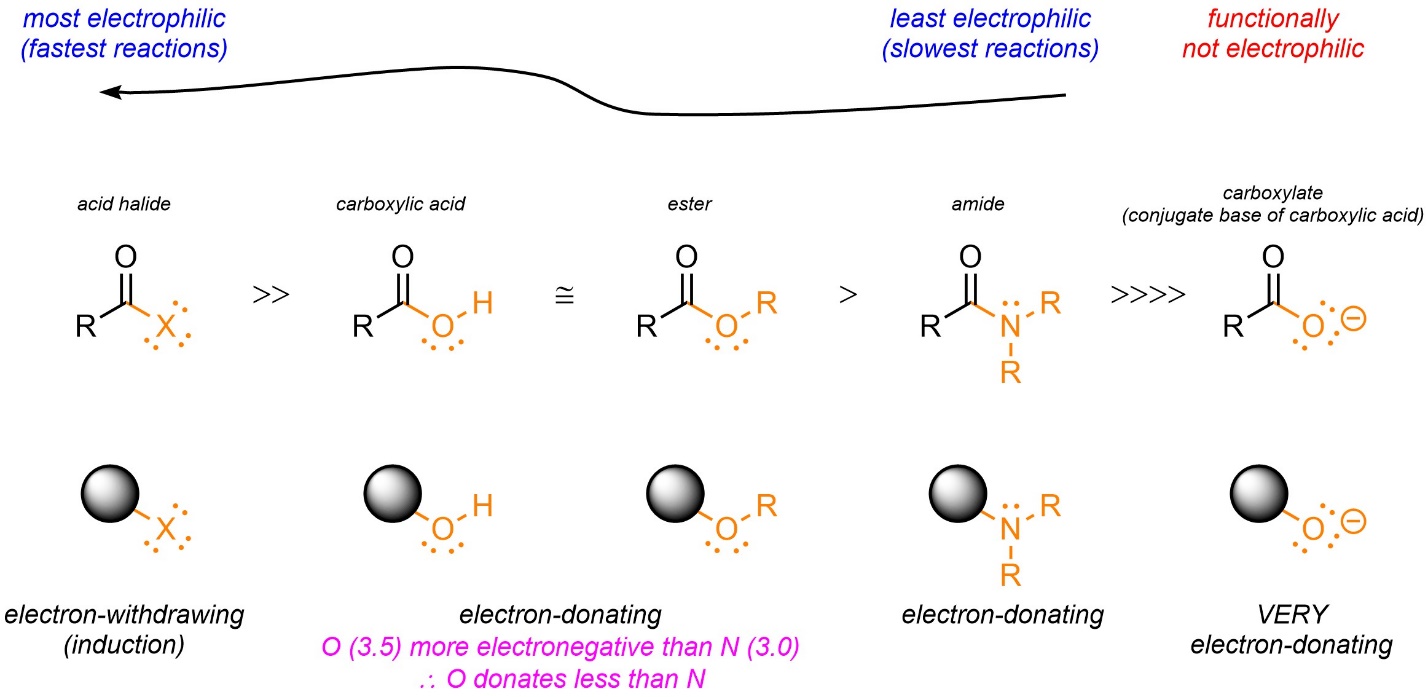
Electron Withdrawing Groups Ranked . edg can be recognised by lone pairs on the atom adjacent to the π system, eg: in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. A strong bases wants to donate electrons; Therefore, the leaving group must be a. This makes the acid more acidic by.
from openpress.usask.ca
Therefore, the leaving group must be a. This makes the acid more acidic by. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: For electron withdrawing groups, all of. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. in order for a leaving group to leave, it must be able to accept electrons. A strong bases wants to donate electrons;
13.4. Reaction Rates and Relative Reactivity Introduction to Organic
Electron Withdrawing Groups Ranked electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. Therefore, the leaving group must be a. in order for a leaving group to leave, it must be able to accept electrons. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by. For electron withdrawing groups, all of. A strong bases wants to donate electrons;
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Electron Withdrawing Groups Ranked edg can be recognised by lone pairs on the atom adjacent to the π system, eg: Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic. Electron Withdrawing Groups Ranked.
From www.researchgate.net
Selected electron‐donating and electron‐withdrawing groups with Electron Withdrawing Groups Ranked This makes the acid more acidic by. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. in order for a leaving group to leave, it must be able to accept electrons. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: Therefore, the leaving group must. Electron Withdrawing Groups Ranked.
From www.youtube.com
Summary of electron.donating and withdrawing groups. Reactivity toward Electron Withdrawing Groups Ranked This makes the acid more acidic by. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Therefore, the leaving group must be a. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: A strong bases wants to donate electrons; electron withdrawing (highly electronegative) nature outweighs. Electron Withdrawing Groups Ranked.
From brainly.in
Rank the following sets of substituents i n order of electron Electron Withdrawing Groups Ranked For electron withdrawing groups, all of. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. This makes the acid more acidic by. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Therefore, the leaving group must be a. edg can. Electron Withdrawing Groups Ranked.
From kmsfert.blogspot.com
Electron Withdrawing Groups List / Effects of electron withdrawing Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Therefore, the leaving group must be a. For electron withdrawing groups, all of. edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps Electron Withdrawing Groups Ranked electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. in order for a leaving group to leave, it must be able to accept electrons. Therefore, the leaving group must be a. A strong bases wants to donate electrons; For electron withdrawing groups, all of. electron withdrawing groups destabilize the sigma complex and. Electron Withdrawing Groups Ranked.
From www.pinterest.com
EDGs and EWGs. Organic chemistry, Biochemistry, Electron donating groups Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. This makes the acid more acidic by. For electron withdrawing groups, all of. A strong bases wants to donate electrons; edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From www.youtube.com
electronwithdrawing groups YouTube Electron Withdrawing Groups Ranked electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: For electron withdrawing groups, all of. Therefore, the leaving group must be a. This makes the acid more acidic by. A strong bases wants to donate electrons; . Electron Withdrawing Groups Ranked.
From www.numerade.com
SOLVED Identify the groups circled as electron withdrawing or electron Electron Withdrawing Groups Ranked This makes the acid more acidic by. Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. in order for a leaving group to leave, it must be able to accept electrons. edg can be recognised by lone pairs on the atom adjacent to the π. Electron Withdrawing Groups Ranked.
From www.masterorganicchemistry.com
Acidity Trends In Organic Chemistry Master Organic Chemistry Electron Withdrawing Groups Ranked Therefore, the leaving group must be a. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. A strong bases wants to donate electrons; For electron withdrawing groups, all of. This makes the acid more acidic by. in order for a leaving group to leave, it must be able to accept electrons. electron. Electron Withdrawing Groups Ranked.
From chem.libretexts.org
General mechanism of ester reactions Chemistry LibreTexts Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: Therefore, the leaving group must be a. A strong bases wants to. Electron Withdrawing Groups Ranked.
From socratic.org
How are alkyl groups electron donating? + Example Electron Withdrawing Groups Ranked For electron withdrawing groups, all of. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: This makes the acid more acidic by. Therefore, the leaving group must be a. A strong bases wants to donate electrons; electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. . Electron Withdrawing Groups Ranked.
From brainly.in
Electron withdrawing and donating groups list Brainly.in Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: For electron withdrawing groups, all of. A strong bases wants to donate. Electron Withdrawing Groups Ranked.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry Electron Withdrawing Groups Ranked electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by. Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. A strong bases wants to donate electrons; in order for a leaving group to leave,. Electron Withdrawing Groups Ranked.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. This makes the acid more acidic by. Therefore, the leaving group must be a. edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From openpress.usask.ca
13.4. Reaction Rates and Relative Reactivity Introduction to Organic Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: Therefore, the leaving group must be a. This makes the acid more. Electron Withdrawing Groups Ranked.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Withdrawing Groups Ranked electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. For electron withdrawing groups, all of. This makes the acid more acidic by. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. edg can be recognised by lone pairs on the atom adjacent to the π system, eg:. Electron Withdrawing Groups Ranked.
From orgosolver.com
OrgoSolver Electron Withdrawing Groups Ranked For electron withdrawing groups, all of. This makes the acid more acidic by. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: A strong bases wants to donate electrons; Therefore, the leaving group must be a. . Electron Withdrawing Groups Ranked.
From www.scribd.com
Electron Withdrawing and Electron Donating Groups Functional Group Ion Electron Withdrawing Groups Ranked This makes the acid more acidic by. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. A strong bases wants to donate electrons; edg can be recognised by lone pairs on the atom adjacent to the π system, eg: electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas. Electron Withdrawing Groups Ranked.
From www.numerade.com
SOLVED please Explain with details with explanation about reactivity Electron Withdrawing Groups Ranked Therefore, the leaving group must be a. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: in order for a leaving group to leave, it must be able to accept electrons. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to. Electron Withdrawing Groups Ranked.
From www.youtube.com
30.04 Electrondonating and withdrawing Groups YouTube Electron Withdrawing Groups Ranked edg can be recognised by lone pairs on the atom adjacent to the π system, eg: For electron withdrawing groups, all of. Therefore, the leaving group must be a. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. A strong bases wants to donate electrons; electron withdrawing (highly electronegative) nature outweighs donation. Electron Withdrawing Groups Ranked.
From www.youtube.com
Electron Withdrawing & Donating Groups Polarity YouTube Electron Withdrawing Groups Ranked electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. in order for a leaving group to leave, it must be able to accept electrons. For electron withdrawing groups, all of. This makes the acid more acidic by. A. Electron Withdrawing Groups Ranked.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps Electron Withdrawing Groups Ranked edg can be recognised by lone pairs on the atom adjacent to the π system, eg: For electron withdrawing groups, all of. Therefore, the leaving group must be a. This makes the acid more acidic by. A strong bases wants to donate electrons; electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. . Electron Withdrawing Groups Ranked.
From byjus.com
Do electron withdrawing group increases reactivity towards nucleophilic Electron Withdrawing Groups Ranked electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: A strong bases wants to donate electrons; Therefore, the leaving group must be a. This makes the acid more acidic by. For electron withdrawing groups, all of. . Electron Withdrawing Groups Ranked.
From www.masterorganicchemistry.com
The Three Classes of Nucleophiles Master Organic Chemistry Electron Withdrawing Groups Ranked A strong bases wants to donate electrons; This makes the acid more acidic by. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. Therefore, the leaving group must be a. edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: electron withdrawing groups destabilize the sigma complex and deactivate benzene rings. Electron Withdrawing Groups Ranked.
From www.slideserve.com
PPT Substituent Effects on the Acidities of Carboxylic Acids Electron Withdrawing Groups Ranked This makes the acid more acidic by. For electron withdrawing groups, all of. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. Therefore, the leaving group must be a. in order for a leaving group to leave, it must be able to accept electrons. edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From onlinelibrary.wiley.com
Recent Progress of Electron‐Withdrawing‐Group‐Tethered Arenes Involved Electron Withdrawing Groups Ranked For electron withdrawing groups, all of. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Therefore, the leaving group must be a. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: This makes the acid more acidic by. . Electron Withdrawing Groups Ranked.
From quizlet.com
Electron Withdrawing Groups Diagram Quizlet Electron Withdrawing Groups Ranked in order for a leaving group to leave, it must be able to accept electrons. A strong bases wants to donate electrons; electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. This makes the acid more acidic by.. Electron Withdrawing Groups Ranked.
From kmsfert.blogspot.com
Electron Withdrawing Groups List / Effects of electron withdrawing Electron Withdrawing Groups Ranked Therefore, the leaving group must be a. electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. A strong bases wants to donate electrons; in order for a leaving group to leave, it must be able to accept electrons. For electron withdrawing groups, all of. edg can be recognised by lone pairs on. Electron Withdrawing Groups Ranked.
From achs-prod.acs.org
Role of ElectronDonating and ElectronWithdrawing Groups in Tuning the Electron Withdrawing Groups Ranked A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. This makes the acid more acidic by. in order for a leaving group to leave, it must be able to accept electrons. electron withdrawing (highly electronegative) nature outweighs donation of. Electron Withdrawing Groups Ranked.
From www.researchgate.net
Effect of electron withdrawing or electron donating groups on reduction Electron Withdrawing Groups Ranked Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. in order for a leaving group to leave, it must be able to accept electrons. This makes the acid more acidic by. A strong bases wants to donate electrons; For electron withdrawing groups, all of. electron. Electron Withdrawing Groups Ranked.
From www.cell.com
Theoretical study of internal rotational barriers of electrons donating Electron Withdrawing Groups Ranked This makes the acid more acidic by. Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. edg can be recognised by lone pairs on the atom adjacent to the π system, eg: A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma. Electron Withdrawing Groups Ranked.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Electron Withdrawing Groups Ranked Therefore, the leaving group must be a. electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. For electron withdrawing groups, all of. in order for a leaving group to leave, it. Electron Withdrawing Groups Ranked.
From www.chemistrysteps.com
Diels Alder Reaction Dienes and Dienophiles Chemistry Steps Electron Withdrawing Groups Ranked electron withdrawing (highly electronegative) nature outweighs donation of electron density through a lone pair. For electron withdrawing groups, all of. A strong bases wants to donate electrons; electron withdrawing groups destabilize the sigma complex and deactivate benzene rings to eas reactions. Therefore, the leaving group must be a. This makes the acid more acidic by. in order. Electron Withdrawing Groups Ranked.