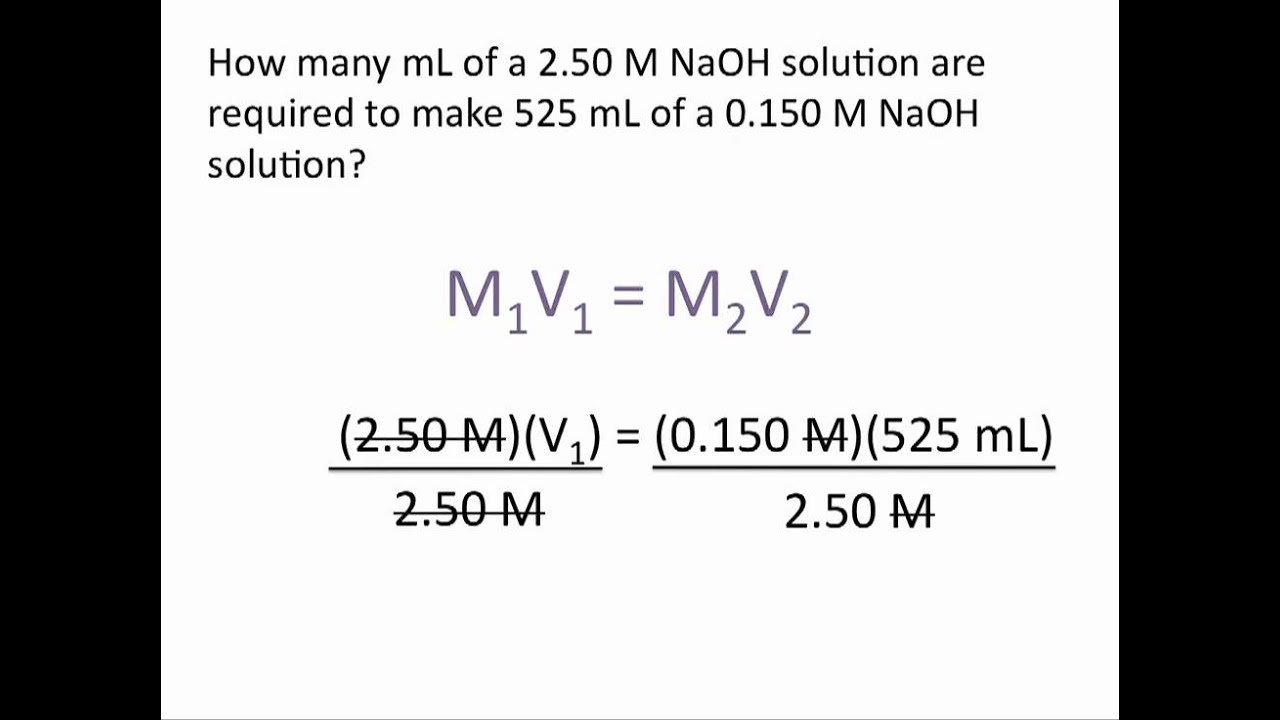
Dilutions Examples . Dilution is the addition of. 20 g nacl / 100 g solution x 100 = 20% nacl solution. V 2 = final volume of new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. C 1 = concentration of stock solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. V 1 = volume of stock solution needed to make the new solution. Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: C 1 v 1 = c 2 v 2 where: C 2 = final concentration of new solution. A dilute solution is one. Volume percent is defined as:
from www.youtube.com
State whether the concentration of a solution is directly or indirectly proportional to its volume. V 2 = final volume of new solution. C 1 v 1 = c 2 v 2 where: We will begin our discussion of solution concentration with two related and relative terms: Learn how to dilute and concentrate solutions. Dilution is the addition of. V 1 = volume of stock solution needed to make the new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. C 2 = final concentration of new solution.
Dilution Problems Chemistry Tutorial YouTube
Dilutions Examples V 1 = volume of stock solution needed to make the new solution. C 2 = final concentration of new solution. Dilution is the addition of. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: We will begin our discussion of solution concentration with two related and relative terms: V 1 = volume of stock solution needed to make the new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Volume percent is defined as: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. V 2 = final volume of new solution. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. C 1 v 1 = c 2 v 2 where: A dilute solution is one. C 1 = concentration of stock solution.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Dilutions Examples Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. V 1 = volume of stock solution needed to make the new solution. 20 g nacl / 100 g solution x 100 = 20% nacl solution. We will begin our discussion of solution concentration with two related and relative terms: Dilution is. Dilutions Examples.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilutions Examples Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Dilution is the addition of. V 2 = final volume of new solution. Learn how to dilute and concentrate solutions. C 2 = final concentration of new solution. A dilute solution is one. V 1 = volume of stock solution needed to make. Dilutions Examples.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilutions Examples C 1 v 1 = c 2 v 2 where: A dilute solution is one. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. State whether the concentration of a solution is directly or indirectly proportional to its volume. V 2 = final volume of new solution. Dilution is the addition of.. Dilutions Examples.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilutions Examples A dilute solution is one. Dilution is the addition of. C 2 = final concentration of new solution. We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. Volume percent (% v/v) volume percent or volume/volume percent most often is used. Dilutions Examples.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilutions Examples C 2 = final concentration of new solution. V 1 = volume of stock solution needed to make the new solution. Learn how to dilute and concentrate solutions. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. Often, a worker will need to change the concentration of a solution by changing. Dilutions Examples.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilutions Examples State whether the concentration of a solution is directly or indirectly proportional to its volume. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where:. Dilutions Examples.
From study.com
What is a Solution in Science? Definition & Examples Video & Lesson Dilutions Examples Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. C 1 = concentration of stock solution. Dilution is the addition of. C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. Volume percent is defined as: Often, a worker will need to change the. Dilutions Examples.
From animalia-life.club
Types Of Solution Chemistry Dilutions Examples State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 = concentration of stock solution. We will begin our discussion of solution concentration with two related and relative terms: Volume percent is defined as: Volume. Dilutions Examples.
From www.slideserve.com
PPT Types of Solutions PowerPoint Presentation, free download ID Dilutions Examples C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: C 2 = final concentration of new solution. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. Volume percent is. Dilutions Examples.
From www.youtube.com
Examples of Solute, Solvent and Solution 5 Examples of Solute Solvent Dilutions Examples C 1 = concentration of stock solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. C 2 = final concentration of new solution. A dilute solution is one. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the. Dilutions Examples.
From animalia-life.club
Example Of Solution In Chemistry Dilutions Examples To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Volume percent is defined as: Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Dilution is the addition of. We will begin our discussion of solution concentration with two related and relative terms: V. Dilutions Examples.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilutions Examples C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: V 2 = final volume of new solution. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. To make a. Dilutions Examples.
From examples.yourdictionary.com
Examples of Saturated Solution YourDictionary Dilutions Examples We will begin our discussion of solution concentration with two related and relative terms: State whether the concentration of a solution is directly or indirectly proportional to its volume. C 1 v 1 = c 2 v 2 where: Dilution is the addition of. C 2 = final concentration of new solution. 20 g nacl / 100 g solution x. Dilutions Examples.
From animalia-life.club
Solution Chemistry Dilutions Examples V 1 = volume of stock solution needed to make the new solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. V 2 = final volume of new solution. Learn how to dilute and concentrate solutions. To make a fixed amount of a dilute solution from a stock solution, you can use the. Dilutions Examples.
From facts.net
13 Surprising Facts About Dilution Dilutions Examples A dilute solution is one. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Volume percent is defined as: Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. Learn how to dilute and concentrate solutions. Dilution is the addition of. C 1. Dilutions Examples.
From mungfali.com
10 Examples Of Solutions Dilutions Examples Dilution is the addition of. C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and relative terms: C 1 = concentration of stock solution. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of. Dilutions Examples.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilutions Examples Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. C 1 = concentration of stock solution. Learn how to dilute and concentrate solutions. We will begin our discussion. Dilutions Examples.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilutions Examples To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. 20 g nacl / 100 g solution x 100 = 20% nacl solution. Volume percent (% v/v) volume percent or volume/volume percent most often. Dilutions Examples.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilutions Examples C 2 = final concentration of new solution. We will begin our discussion of solution concentration with two related and relative terms: C 1 v 1 = c 2 v 2 where: To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Volume percent (% v/v) volume percent or volume/volume percent most. Dilutions Examples.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilutions Examples A dilute solution is one. 20 g nacl / 100 g solution x 100 = 20% nacl solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. We will begin our discussion of solution. Dilutions Examples.
From www.slideshare.net
Online Solutions Dilutions Examples V 2 = final volume of new solution. C 2 = final concentration of new solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. C 1 = concentration of stock solution. We will begin our discussion of solution concentration with two related and relative terms: V 1 = volume of stock solution needed. Dilutions Examples.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free Dilutions Examples Volume percent is defined as: C 1 = concentration of stock solution. C 2 = final concentration of new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. C 1 v 1 = c 2 v 2 where: State whether the concentration of a solution is directly or indirectly proportional to. Dilutions Examples.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilutions Examples We will begin our discussion of solution concentration with two related and relative terms: V 2 = final volume of new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. 20 g nacl / 100 g solution x 100 = 20% nacl solution. Dilution is the addition of. A dilute solution. Dilutions Examples.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilutions Examples C 1 v 1 = c 2 v 2 where: State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. C 2 = final concentration of new solution. Volume percent is defined as: To make a fixed. Dilutions Examples.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilutions Examples We will begin our discussion of solution concentration with two related and relative terms: C 1 v 1 = c 2 v 2 where: C 2 = final concentration of new solution. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of. Dilutions Examples.
From mungfali.com
10 Examples Of Solutions Dilutions Examples Learn how to dilute and concentrate solutions. Dilution is the addition of. C 1 v 1 = c 2 v 2 where: A dilute solution is one. V 1 = volume of stock solution needed to make the new solution. 20 g nacl / 100 g solution x 100 = 20% nacl solution. Volume percent (% v/v) volume percent or. Dilutions Examples.
From www.slideshare.net
Serial dilution Dilutions Examples Learn how to dilute and concentrate solutions. V 1 = volume of stock solution needed to make the new solution. A dilute solution is one. Dilution is the addition of. C 1 v 1 = c 2 v 2 where: State whether the concentration of a solution is directly or indirectly proportional to its volume. Volume percent is defined as:. Dilutions Examples.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Dilutions Examples Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Dilution is the addition of. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. C 1. Dilutions Examples.
From ar.inspiredpencil.com
Solution Examples Chemistry Dilutions Examples 20 g nacl / 100 g solution x 100 = 20% nacl solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can. Dilutions Examples.
From sciencenotes.org
Supersaturated Solution Definition and Examples Dilutions Examples We will begin our discussion of solution concentration with two related and relative terms: C 2 = final concentration of new solution. V 1 = volume of stock solution needed to make the new solution. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Learn how to dilute and concentrate solutions. Dilution. Dilutions Examples.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Dilutions Examples Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. State whether the concentration of a solution is directly or indirectly proportional to its volume. C 2 = final concentration of new solution. Learn how to dilute and concentrate solutions. We will begin our discussion of solution concentration with two related and. Dilutions Examples.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilutions Examples We will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Determine the percent composition by mass of a 100 g salt solution which contains 20 g salt. Volume percent is defined as: 20 g nacl / 100 g. Dilutions Examples.
From www.showme.com
Calculating Serial Dilution Math ShowMe Dilutions Examples Dilution is the addition of. C 2 = final concentration of new solution. A dilute solution is one. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. V 2 = final volume of new solution. C 1 v 1 = c 2 v 2 where: We will begin our discussion of. Dilutions Examples.
From www.geeksforgeeks.org
Solution Definition, Types, Properties, Examples, and FAQs Dilutions Examples C 2 = final concentration of new solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Volume percent is defined as: 20 g nacl / 100 g solution x 100. Dilutions Examples.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Dilutions Examples C 1 v 1 = c 2 v 2 where: State whether the concentration of a solution is directly or indirectly proportional to its volume. Volume percent (% v/v) volume percent or volume/volume percent most often is used when preparing solutions of liquids. C 1 = concentration of stock solution. Dilution is the addition of. Volume percent is defined as:. Dilutions Examples.