Laboratory Information System Validation Procedure . This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in.
from www.semanticscholar.org
The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. It specifies more precisely the.
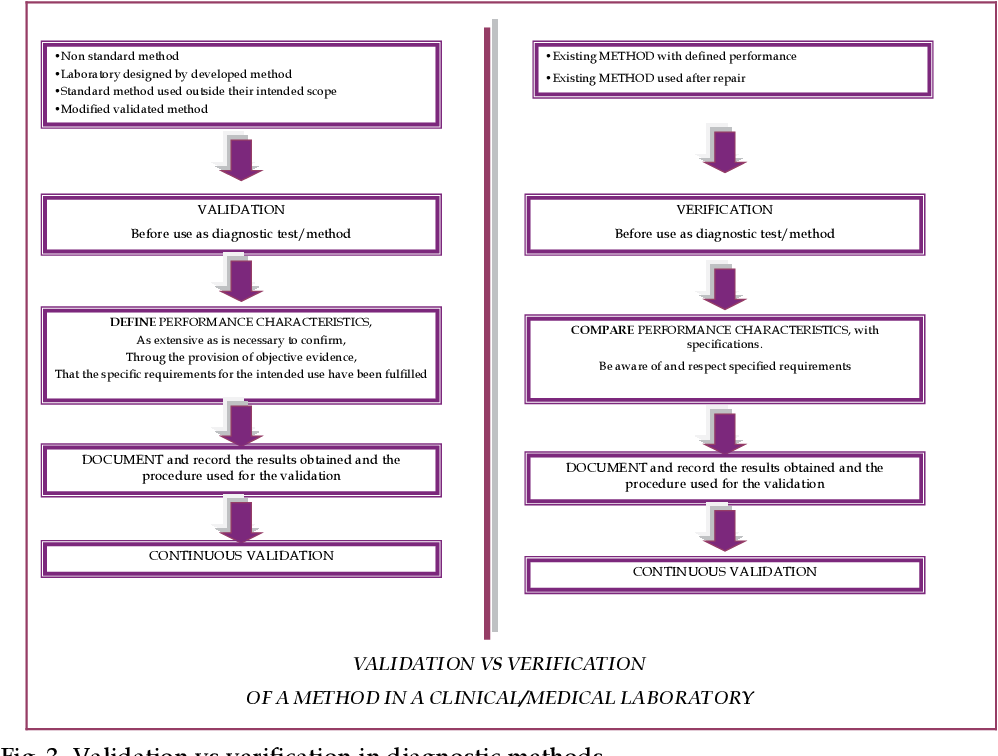
Figure 3 from Procedures for Validation of Diagnostic Methods in
Laboratory Information System Validation Procedure It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. It specifies more precisely the.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Laboratory Information System Validation Procedure Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a. Laboratory Information System Validation Procedure.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Laboratory Information System Validation Procedure The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. The clinical and laboratory standards institute published important factors that should be considered when developing. It specifies more precisely the. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols. Laboratory Information System Validation Procedure.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims).. Laboratory Information System Validation Procedure.
From www.degruyter.com
Validation and verification of examination procedures in medical Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing. Laboratory Information System Validation Procedure.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The clinical and laboratory standards institute published important factors that should be considered when developing. It specifies more precisely the. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). This document provides guidance for. Laboratory Information System Validation Procedure.
From www.slideshare.net
PROCESS VALIDATION Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing.. Laboratory Information System Validation Procedure.
From technology.gov.capital
Laboratory information system validation Technology.Gov.Capital Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. It specifies more precisely the. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. The aim of this study was to establish a protocol for retrospective validation of laboratory. Laboratory Information System Validation Procedure.
From studylib.net
Laboratory Method Validation For any method that will be used for Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. The clinical and laboratory standards institute published important factors that should be considered when developing.. Laboratory Information System Validation Procedure.
From www.slideshare.net
Validation Master Plan Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information. Laboratory Information System Validation Procedure.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a protocol. Laboratory Information System Validation Procedure.
From labos.co
How to Validate a Laboratory Information System LabOS Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of.. Laboratory Information System Validation Procedure.
From www.semanticscholar.org
Figure 3 from Procedures for Validation of Diagnostic Methods in Laboratory Information System Validation Procedure Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing. Laboratory Information System Validation Procedure.
From www.researchgate.net
(PDF) Validation of a laboratory and hospital information system in a Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in.. Laboratory Information System Validation Procedure.
From www.researchgate.net
Flowchart for the optimization procedure and validation. Procedure to Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of.. Laboratory Information System Validation Procedure.
From www.limsforum.com
Guide to Laboratory Information Management System Software Validations Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of.. Laboratory Information System Validation Procedure.
From fivevalidation.com
LIMS Laboratory Information Management System FIVE Validation Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The aim of this study was to establish a protocol. Laboratory Information System Validation Procedure.
From mavink.com
Laboratory Validation Template Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. It specifies more precisely the. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document. Laboratory Information System Validation Procedure.
From www.youtube.com
How to Validate and Verify the Accuracy of your Clinical Laboratory Laboratory Information System Validation Procedure Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The clinical and laboratory standards institute published important factors that should be considered when developing. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. This document provides guidance for developing a protocol for validation. Laboratory Information System Validation Procedure.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. It specifies more precisely the. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Scope 1.1 this guide describes an approach to the validation process for a. Laboratory Information System Validation Procedure.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Laboratory Information System Validation Procedure It specifies more precisely the. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Scope 1.1 this guide describes an approach to the validation. Laboratory Information System Validation Procedure.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The clinical and laboratory standards institute published important factors that should be considered when developing. It specifies more. Laboratory Information System Validation Procedure.
From www.researchgate.net
Contents in a validation procedure in clinical laboratory. Download Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. It specifies more precisely the. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The clinical and laboratory standards institute published important factors that should be. Laboratory Information System Validation Procedure.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. The aim of this study was to establish a protocol for retrospective. Laboratory Information System Validation Procedure.
From www.presentationeze.com
Medical Device Validation Full Details PresentationEZE Laboratory Information System Validation Procedure The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The clinical and laboratory standards institute published important factors that should be considered when developing. This document provides guidance for developing a. Laboratory Information System Validation Procedure.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Laboratory Information System Validation Procedure It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document. Laboratory Information System Validation Procedure.
From kvalito.ch
How is a system validated? Kvalito Laboratory Information System Validation Procedure The clinical and laboratory standards institute published important factors that should be considered when developing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. It specifies more precisely the. Scope 1.1 this. Laboratory Information System Validation Procedure.
From www.slideserve.com
PPT Laboratory Information Management Systems PowerPoint Presentation Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. It specifies more precisely the. Laboratory information systems (lis) play a key role in laboratories. Laboratory Information System Validation Procedure.
From www.scribd.com
Method Validation Report Template 1 Experiment Accuracy And Precision Laboratory Information System Validation Procedure It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. This document provides guidance for. Laboratory Information System Validation Procedure.
From www.globalspec.com
Laboratory Information Management Systems (LIMS) Selection Guide Types Laboratory Information System Validation Procedure This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. It specifies more precisely the. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. Scope 1.1 this guide describes an approach to the validation. Laboratory Information System Validation Procedure.
From www.collidu.com
Laboratory Information Management System (LIMS) PowerPoint and Google Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. It specifies more precisely the. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in.. Laboratory Information System Validation Procedure.
From studylib.net
Validation of Computerized Laboratory Systems Laboratory Information System Validation Procedure It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. This document provides guidance for developing a protocol for validation of the laboratory information system (lis), as well as protocols for assessing the dependability of. Scope 1.1 this guide describes an approach to the validation process for a laboratory information. Laboratory Information System Validation Procedure.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Laboratory Information System Validation Procedure The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. The clinical and laboratory standards institute published important factors that should be considered when developing. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. It specifies more precisely the. Scope 1.1 this. Laboratory Information System Validation Procedure.
From www.uslegalforms.com
AUTO8P Protocols To Validate Laboratory Information Systems Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). The clinical and laboratory standards institute published important factors that should be considered when developing. This document provides guidance for developing a protocol for validation. Laboratory Information System Validation Procedure.
From blog.labtag.com
Benefits of a Laboratory Information Management System Labtag Blog Laboratory Information System Validation Procedure It specifies more precisely the. The clinical and laboratory standards institute published important factors that should be considered when developing. Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The aim of this study was to establish a protocol for retrospective validation of laboratory and hospital information systems in. This document provides. Laboratory Information System Validation Procedure.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Laboratory Information System Validation Procedure Laboratory information systems (lis) play a key role in laboratories meeting quality standards, decreasing transcription errors, reducing. The clinical and laboratory standards institute published important factors that should be considered when developing. Scope 1.1 this guide describes an approach to the validation process for a laboratory information management system (lims). It specifies more precisely the. The aim of this study. Laboratory Information System Validation Procedure.