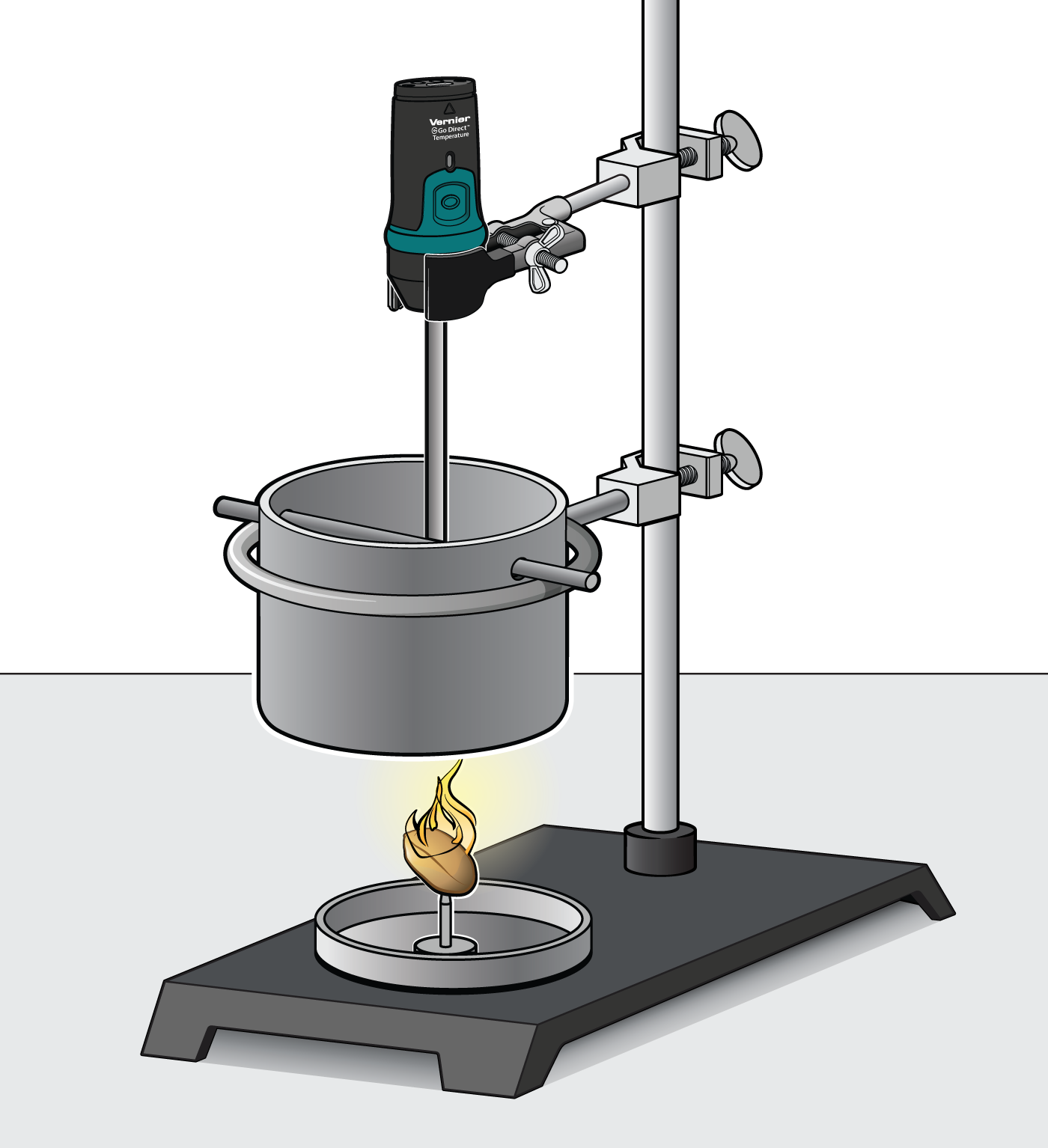
Burning Food Lab Answers . The distance between the burning food and the test tube. From the measured temperature change,. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. energy content of food. what is the source of the energy? “burn” calories, but we can also burn calories (literally) by setting food on fire! If you check the label of any package of foodstuffs, you will see a nutritional. calculate the change in temperature caused by the burning food sample. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. It comes from the food that we eat. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water.
from www.vernier.com
From the measured temperature change,. If you check the label of any package of foodstuffs, you will see a nutritional. what is the source of the energy? calculate the change in temperature caused by the burning food sample. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It comes from the food that we eat. “burn” calories, but we can also burn calories (literally) by setting food on fire! in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. energy content of food.
Investigating the Energy Content of Foods > Experiment 6 from Investigating Chemistry through
Burning Food Lab Answers If you check the label of any package of foodstuffs, you will see a nutritional. what is the source of the energy? By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. “burn” calories, but we can also burn calories (literally) by setting food on fire! If you check the label of any package of foodstuffs, you will see a nutritional. energy content of food. It comes from the food that we eat. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. calculate the change in temperature caused by the burning food sample. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. From the measured temperature change,. The distance between the burning food and the test tube.
From www.youtube.com
Burning Food Lab YouTube Burning Food Lab Answers energy content of food. From the measured temperature change,. “burn” calories, but we can also burn calories (literally) by setting food on fire! in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. calculate the change in temperature caused by the burning food sample. It. Burning Food Lab Answers.
From childhealthpolicy.vumc.org
🌈 Burning food experiment results. Lab Report 18. 20221025 Burning Food Lab Answers what is the source of the energy? The distance between the burning food and the test tube. calculate the change in temperature caused by the burning food sample. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. energy content of food. If you check the label. Burning Food Lab Answers.
From devonnewsrichardson.blogspot.com
Specific Heat and Calorimetry Worksheet Answer Key Burning Food Lab Answers calculate the change in temperature caused by the burning food sample. “burn” calories, but we can also burn calories (literally) by setting food on fire! From the measured temperature change,. energy content of food. The distance between the burning food and the test tube. what is the source of the energy? in this practical, students burn. Burning Food Lab Answers.
From studylib.net
LAB Food Lab Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! If you check the label of any package of foodstuffs, you will see a nutritional. It comes from the food that we eat. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. in this practical, students. Burning Food Lab Answers.
From www.chegg.com
Solved Lab 6Food Calorimetry Lab Page 50 Reactions that Burning Food Lab Answers energy content of food. The distance between the burning food and the test tube. From the measured temperature change,. It comes from the food that we eat. “burn” calories, but we can also burn calories (literally) by setting food on fire! in this practical, students burn a sample of a foodstuff of known mass, heating a known volume. Burning Food Lab Answers.
From www.seriouseats.com
The Book Has Arrived The Food Lab Burning Food Lab Answers energy content of food. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. calculate the change in temperature caused by the burning food sample. If you check the label of any package of foodstuffs, you will see a nutritional. The distance between the burning food and the. Burning Food Lab Answers.
From www.preproom.org
Releasing Energy in Food Science Practical Expiriment used in School and Education Burning Food Lab Answers calculate the change in temperature caused by the burning food sample. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. energy content of food. From the measured temperature change,. “burn” calories, but we can also burn calories (literally) by setting food on fire! It comes from the food that. Burning Food Lab Answers.
From www.studocu.com
LAB18106 LAB Ankush Sharma 07/08/ Lab 18 Calorimetry and Calculation “Burning Food. Where Burning Food Lab Answers energy content of food. If you check the label of any package of foodstuffs, you will see a nutritional. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. From the measured temperature change,. “burn” calories, but we can also burn calories (literally) by setting food on fire! . Burning Food Lab Answers.
From www.youtube.com
How much energy is in our food? Crisp burning lab practical with Mr Pash! YouTube Burning Food Lab Answers If you check the label of any package of foodstuffs, you will see a nutritional. It comes from the food that we eat. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. From the measured temperature change,. energy content of food. The distance between the burning food and. Burning Food Lab Answers.
From schoolworkhelper.net
Lab Answers Energy from Burning Food SchoolWorkHelper Burning Food Lab Answers in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. If you check the label of any package of foodstuffs, you will see a nutritional. From the measured temperature change,. The distance between the burning food and the test tube. “burn” calories, but we can also burn. Burning Food Lab Answers.
From www.youtube.com
Burning Food Experiment food experiments food burning burning food YouTube Burning Food Lab Answers in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. energy content of food. what is the source of the energy? in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. The distance between the. Burning Food Lab Answers.
From studylib.net
Foods Lab Rules Burning Food Lab Answers From the measured temperature change,. “burn” calories, but we can also burn calories (literally) by setting food on fire! what is the source of the energy? If you check the label of any package of foodstuffs, you will see a nutritional. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light.. Burning Food Lab Answers.
From schoolworkhelper.net
Lab Answers Energy from Burning Food SchoolWorkHelper Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. From the measured temperature change,. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn.. Burning Food Lab Answers.
From childhealthpolicy.vumc.org
🌈 Burning food experiment results. Lab Report 18. 20221025 Burning Food Lab Answers energy content of food. From the measured temperature change,. what is the source of the energy? The distance between the burning food and the test tube. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. in this experiment, you will determine the energy released (in kj/g) as various. Burning Food Lab Answers.
From studyschoolminorship.z21.web.core.windows.net
Safety In Laboratory Pdf Burning Food Lab Answers The distance between the burning food and the test tube. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It comes from the food that we. Burning Food Lab Answers.
From www.bostonmagazine.com
Holy Smokes! Why Chefs Are Burning Food on Purpose Burning Food Lab Answers in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. From the measured temperature change,. The distance between the burning food and the test tube. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. energy content of. Burning Food Lab Answers.
From www.academia.edu
(PDF) Lab Answers Energy from Burning Food Burning Food Lab Answers in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. what is the source of the energy? The distance between the burning food and the test tube. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. in this experiment,. Burning Food Lab Answers.
From www.youtube.com
Burning Foods YouTube Burning Food Lab Answers The distance between the burning food and the test tube. what is the source of the energy? From the measured temperature change,. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It comes from the food that we eat. By burning pieces of food, the chemical energy stored. Burning Food Lab Answers.
From www.youtube.com
Burning foods practical IGCSE Biology YouTube Burning Food Lab Answers in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It comes from the food that we eat. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. energy content of food. If you check the label of any package of. Burning Food Lab Answers.
From childhealthpolicy.vumc.org
🌈 Burning food experiment results. Lab Report 18. 20221025 Burning Food Lab Answers in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. what is the source of the energy? If you check the label of any package of foodstuffs, you will see a nutritional. The distance between the burning food and the test tube. From the measured temperature change,. calculate. Burning Food Lab Answers.
From www.studocu.com
Lab 6 food energy for Kathryn D. Burns Lab 6 Energy in food Objective For this experiment Burning Food Lab Answers in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. The distance between the burning food and the test tube. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. “burn” calories, but we can also burn calories (literally) by setting food. Burning Food Lab Answers.
From www.chegg.com
Solved EXPERIMENT 1 DETERMINING THE ENERGY IN FOOD Data Burning Food Lab Answers By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. what is the source of the energy? From the measured temperature change,. energy content of food. If. Burning Food Lab Answers.
From schoolworkhelper.net
Energy Content of Food Lab Report Answers SchoolWorkHelper Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! If you check the label of any package of foodstuffs, you will see a nutritional. From the measured temperature change,. energy content of food. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. calculate the. Burning Food Lab Answers.
From www.vernier.com
Investigating the Energy Content of Foods > Experiment 6 from Investigating Chemistry through Burning Food Lab Answers If you check the label of any package of foodstuffs, you will see a nutritional. It comes from the food that we eat. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. what is the source of the energy? calculate the change in temperature caused by the. Burning Food Lab Answers.
From www.markedbyteachers.com
Experiment. How much energy is there in some foods that you eat? GCSE Science Marked by Burning Food Lab Answers From the measured temperature change,. calculate the change in temperature caused by the burning food sample. The distance between the burning food and the test tube. By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. “burn” calories, but we can also burn calories (literally) by setting food on fire! . Burning Food Lab Answers.
From www.studocu.com
Lab energy burning food into energy Burning food into energy Table number 1 Lab Results Burning Food Lab Answers By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. energy content of food. From the measured temperature change,. It comes from the food that we eat. . Burning Food Lab Answers.
From studylib.net
Unit 1A. Burning Candle Lab Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! If you check the label of any package of foodstuffs, you will see a nutritional. The distance between the burning food and the test tube. calculate the change in temperature caused by the burning food sample. what is the source of the energy? . Burning Food Lab Answers.
From www.studocu.com
Burning Food Formal Lab CHEM 100 Heat of Food Lab The purpose of this lab is to determine this Burning Food Lab Answers in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. If you check the label of any package of foodstuffs, you will see a nutritional. The distance between the burning food and the test tube. what is the source of the energy? From the measured temperature. Burning Food Lab Answers.
From www.tes.com
Energy in Food (Burning Food) Year 7 Double Lesson PowerPoint (KS3 7Ia) Energy topic Teaching Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. what is the source of the energy? calculate the change in temperature caused by the burning food sample. If you check the label of any package. Burning Food Lab Answers.
From www.tes.com
Energy in Food (Burning Food) Year 7 Double Lesson PowerPoint (KS3 7Ia) Energy topic Teaching Burning Food Lab Answers calculate the change in temperature caused by the burning food sample. If you check the label of any package of foodstuffs, you will see a nutritional. It comes from the food that we eat. The distance between the burning food and the test tube. what is the source of the energy? By burning pieces of food, the chemical. Burning Food Lab Answers.
From tukioka-clinic.com
😎 Burning fuels experiment results. Candle Burning Experiment. 20190220 Burning Food Lab Answers “burn” calories, but we can also burn calories (literally) by setting food on fire! By burning pieces of food, the chemical energy stored in molecular bonds is released as heat and light. It comes from the food that we eat. calculate the change in temperature caused by the burning food sample. If you check the label of any package. Burning Food Lab Answers.
From quizizz.com
Experiment Energy in Food Physics Quizizz Burning Food Lab Answers calculate the change in temperature caused by the burning food sample. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. The distance between the burning food and the test tube. “burn” calories, but we can also burn calories (literally) by setting food on fire! From. Burning Food Lab Answers.
From agclassroom.org
FoodMASTER Middle Energy Balance Burning Food Lab Answers calculate the change in temperature caused by the burning food sample. From the measured temperature change,. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water.. Burning Food Lab Answers.
From chart-studio.plotly.com
Burning Food Practical bar chart made by Adgethi1 plotly Burning Food Lab Answers If you check the label of any package of foodstuffs, you will see a nutritional. calculate the change in temperature caused by the burning food sample. what is the source of the energy? It comes from the food that we eat. From the measured temperature change,. “burn” calories, but we can also burn calories (literally) by setting food. Burning Food Lab Answers.
From dxolvbibq.blob.core.windows.net
Examples Of Food Laboratory at Woodrow Fray blog Burning Food Lab Answers in this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. It comes from the food that we eat. in this experiment, you will determine the energy released (in kj/g) as various foods, such as cashews, marshmallows, peanuts, and popcorn, burn. energy content of food. calculate the change. Burning Food Lab Answers.