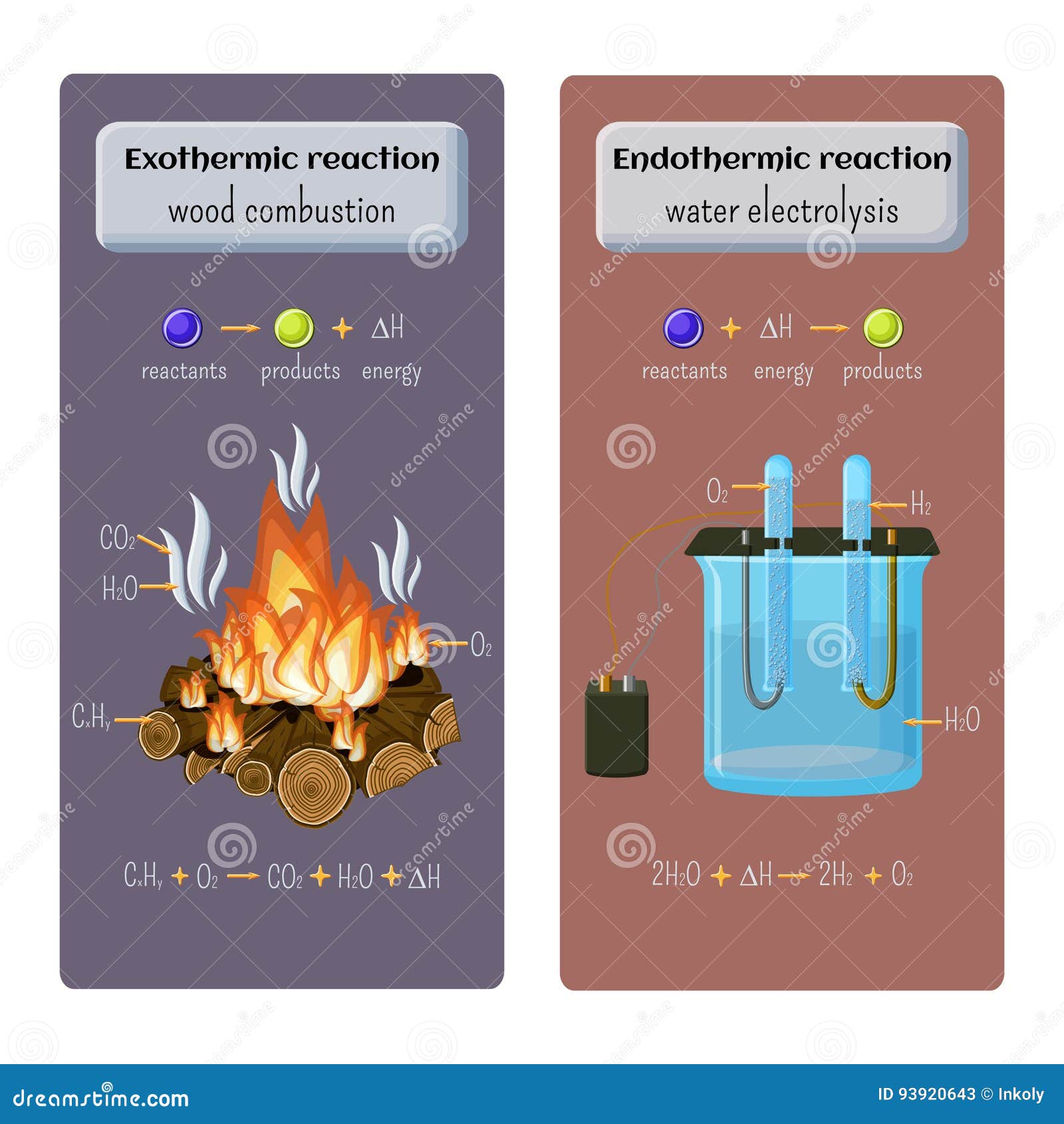
Endothermic Reaction Of Electrolysis . an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Pure water does not conduct electricity and requires. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. exothermic and endothermic reactions. The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: \text{mol}\) of calcium carbonate decomposes into \(1 \: Because heat is absorbed, endothermic. In endothermic and exothermic reactions, energy can be thought of as. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Other chemical reactions release energy in the form of heat, light, or sound.
from www.dreamstime.com
some examples of endothermic reactions are: Pure water does not conduct electricity and requires. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. exothermic and endothermic reactions. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). The reaction between ethanoic acid and sodium carbonate. Other chemical reactions release energy in the form of heat, light, or sound. In endothermic and exothermic reactions, energy can be thought of as. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.
Types of Chemical Reaction. Exothermic Wood Combustion and Endothermic Water Electrolysis
Endothermic Reaction Of Electrolysis The reaction between ethanoic acid and sodium carbonate. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic. \text{mol}\) of calcium carbonate decomposes into \(1 \: some examples of endothermic reactions are: In endothermic and exothermic reactions, energy can be thought of as. Pure water does not conduct electricity and requires. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. exothermic and endothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Of Electrolysis Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. some examples of endothermic reactions are: Pure water does not conduct electricity and. Endothermic Reaction Of Electrolysis.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Of Electrolysis The reaction between ethanoic acid and sodium carbonate. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. . Endothermic Reaction Of Electrolysis.
From www.dreamstime.com
Types of Chemical Reaction. Exothermic Wood Combustion and Endothermic Water Electrolysis Endothermic Reaction Of Electrolysis \text{mol}\) of calcium carbonate decomposes into \(1 \: Pure water does not conduct electricity and requires. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. some examples of endothermic reactions are: exothermic and endothermic reactions. Because heat is absorbed, endothermic. The reaction between ethanoic acid and sodium. Endothermic Reaction Of Electrolysis.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Of Electrolysis Other chemical reactions release energy in the form of heat, light, or sound. \text{mol}\) of calcium carbonate decomposes into \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic. . Endothermic Reaction Of Electrolysis.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Of Electrolysis Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other. Endothermic Reaction Of Electrolysis.
From www.youtube.com
Electrolysis of water, Exothermic, endothermic, displacement, and double displacement reactions Endothermic Reaction Of Electrolysis endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of calcium carbonate decomposes into \(1 \: Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Other chemical reactions release energy in the form of. Endothermic Reaction Of Electrolysis.
From slideplayer.com
Thermochemistry CHAPTER ppt download Endothermic Reaction Of Electrolysis an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. In endothermic and exothermic reactions, energy can be thought of as. Pure water does not conduct electricity and requires. some examples of endothermic reactions. Endothermic Reaction Of Electrolysis.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Of Electrolysis exothermic and endothermic reactions. Because heat is absorbed, endothermic. The reaction between ethanoic acid and sodium carbonate. \text{mol}\) of calcium carbonate decomposes into \(1 \: endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Other chemical reactions release energy in the form of heat, light, or sound. some examples. Endothermic Reaction Of Electrolysis.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes Endothermic Reaction Of Electrolysis The reaction between ethanoic acid and sodium carbonate. exothermic and endothermic reactions. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. \text{mol}\) of calcium carbonate decomposes into \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Pure. Endothermic Reaction Of Electrolysis.
From www.slideshare.net
Tang 04 electrolysis 2 Endothermic Reaction Of Electrolysis exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be thought of as. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. some examples of endothermic reactions. Endothermic Reaction Of Electrolysis.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Endothermic Reaction Of Electrolysis an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Other chemical reactions release energy in the form of heat, light, or sound. Because heat is absorbed, endothermic. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions may occur spontaneously and result in higher. Endothermic Reaction Of Electrolysis.
From www.numerade.com
SOLVED classify the following chemical reaction exothermic or endothermic first electrolysis of Endothermic Reaction Of Electrolysis Because heat is absorbed, endothermic. Pure water does not conduct electricity and requires. \text{mol}\) of calcium carbonate decomposes into \(1 \: In endothermic and exothermic reactions, energy can be thought of as. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. endothermic reactions are characterized by positive heat flow (into the reaction) and. Endothermic Reaction Of Electrolysis.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Chemistry Endothermic Reaction Of Electrolysis endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). exothermic and endothermic reactions. The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. some examples of endothermic reactions are: In endothermic. Endothermic Reaction Of Electrolysis.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Of Electrolysis Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be thought of as. endothermic reactions are characterized by. Endothermic Reaction Of Electrolysis.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Of Electrolysis Pure water does not conduct electricity and requires. Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. \text{mol}\) of calcium carbonate decomposes into \(1 \: . Endothermic Reaction Of Electrolysis.
From www.studypool.com
SOLUTION Stoichiometry concentration exo and endothermic enthalpy reactions electrolysis Endothermic Reaction Of Electrolysis \text{mol}\) of calcium carbonate decomposes into \(1 \: some examples of endothermic reactions are: Other chemical reactions release energy in the form of heat, light, or sound. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). The reaction between ethanoic acid and sodium carbonate. exothermic and endothermic reactions. . Endothermic Reaction Of Electrolysis.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Reaction Of Electrolysis Pure water does not conduct electricity and requires. Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other. Endothermic Reaction Of Electrolysis.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Of Electrolysis Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. The reaction between ethanoic acid and sodium carbonate. In endothermic and exothermic reactions, energy can be thought. Endothermic Reaction Of Electrolysis.
From ppt-online.org
Thermal Energy, Chemical Energy презентация онлайн Endothermic Reaction Of Electrolysis revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). In endothermic and exothermic reactions, energy can be thought of as. Because heat is absorbed, endothermic. Other chemical reactions release energy in. Endothermic Reaction Of Electrolysis.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Endothermic Reaction Of Electrolysis Because heat is absorbed, endothermic. exothermic and endothermic reactions. some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. In endothermic and exothermic reactions, energy can be thought of as. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or. Endothermic Reaction Of Electrolysis.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Of Electrolysis Other chemical reactions release energy in the form of heat, light, or sound. In endothermic and exothermic reactions, energy can be thought of as. some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. Exothermic reactions may occur spontaneously and result in higher. Endothermic Reaction Of Electrolysis.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Of Electrolysis \text{mol}\) of calcium carbonate decomposes into \(1 \: an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In endothermic and exothermic reactions, energy can be thought of as. The reaction between ethanoic acid and sodium carbonate. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system.. Endothermic Reaction Of Electrolysis.
From userlistvuleucoplast.z13.web.core.windows.net
Energy Diagram For Exothermic Reaction Endothermic Reaction Of Electrolysis Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Because heat is absorbed, endothermic. endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. an endothermic reaction. Endothermic Reaction Of Electrolysis.
From www.coursehero.com
[Solved] A model of an endothermic reaction and an exothermic reaction Course Hero Endothermic Reaction Of Electrolysis some examples of endothermic reactions are: endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Pure water does not conduct electricity and requires. \text{mol}\) of calcium carbonate decomposes into \(1 \: an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. revise and understand. Endothermic Reaction Of Electrolysis.
From www.freepik.com
Premium Vector Electrolysis process vector illustration diagram Endothermic Reaction Of Electrolysis Pure water does not conduct electricity and requires. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. some examples of endothermic reactions are: Because heat is absorbed, endothermic. The reaction between ethanoic acid. Endothermic Reaction Of Electrolysis.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Of Electrolysis Pure water does not conduct electricity and requires. exothermic and endothermic reactions. Because heat is absorbed, endothermic. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium. Endothermic Reaction Of Electrolysis.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Of Electrolysis Pure water does not conduct electricity and requires. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. \text{mol}\) of calcium carbonate decomposes into \(1 \: exothermic and endothermic reactions. some examples of endothermic reactions are: Other chemical reactions release energy in the form of. Endothermic Reaction Of Electrolysis.
From www.slideserve.com
PPT Endothermic & Exothermic Reactions PowerPoint Presentation ID3030634 Endothermic Reaction Of Electrolysis endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). some examples of endothermic reactions are: Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. exothermic and endothermic. Endothermic Reaction Of Electrolysis.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction Of Electrolysis In endothermic and exothermic reactions, energy can be thought of as. Other chemical reactions release energy in the form of heat, light, or sound. Because heat is absorbed, endothermic. Pure water does not conduct electricity and requires. exothermic and endothermic reactions. The reaction between ethanoic acid and sodium carbonate. endothermic reactions are characterized by positive heat flow (into. Endothermic Reaction Of Electrolysis.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Of Electrolysis In endothermic and exothermic reactions, energy can be thought of as. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. Pure water does not conduct electricity and requires. \text{mol}\) of calcium carbonate decomposes into \(1 \: endothermic reactions are characterized by positive heat flow (into. Endothermic Reaction Of Electrolysis.
From slideplayer.com
Energy & Chemical Reactions ppt download Endothermic Reaction Of Electrolysis some examples of endothermic reactions are: endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). \text{mol}\) of calcium carbonate decomposes into \(1 \: Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. exothermic and endothermic reactions. an endothermic reaction. Endothermic Reaction Of Electrolysis.
From ppt-online.org
Electrolysis презентация онлайн Endothermic Reaction Of Electrolysis an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Pure water does not conduct electricity and requires. In endothermic and exothermic reactions, energy can be thought of as. Other chemical reactions release energy in the form. Endothermic Reaction Of Electrolysis.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Coordinate Diagram Endothermic Reaction Of Electrolysis some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or. The reaction between ethanoic acid and sodium carbonate. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy. Endothermic Reaction Of Electrolysis.
From slideplayer.com
INDUSTRIAL CHEMISTRY CHEM327 BY DR. GULAM ABBAS. ppt download Endothermic Reaction Of Electrolysis \text{mol}\) of calcium carbonate decomposes into \(1 \: Pure water does not conduct electricity and requires. an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. exothermic and endothermic reactions. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. The reaction between ethanoic acid and. Endothermic Reaction Of Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic... Download Scientific Diagram Endothermic Reaction Of Electrolysis an endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Pure water does not conduct electricity and requires. \text{mol}\) of calcium carbonate decomposes into \(1 \: Other chemical reactions release energy in the form of heat, light, or sound. In endothermic and exothermic reactions, energy can be thought of as. exothermic and endothermic reactions.. Endothermic Reaction Of Electrolysis.