How Do H+ Ions Exist In Nature . However, in water, h+ ions cannot exist. In solution, meaning water, a lone. typically, chemists write the acidic ion in solution as simply h+. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. Hcl h c l only.
from www.youtube.com
hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. typically, chemists write the acidic ion in solution as simply h+. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. However, in water, h+ ions cannot exist. Hcl h c l only.
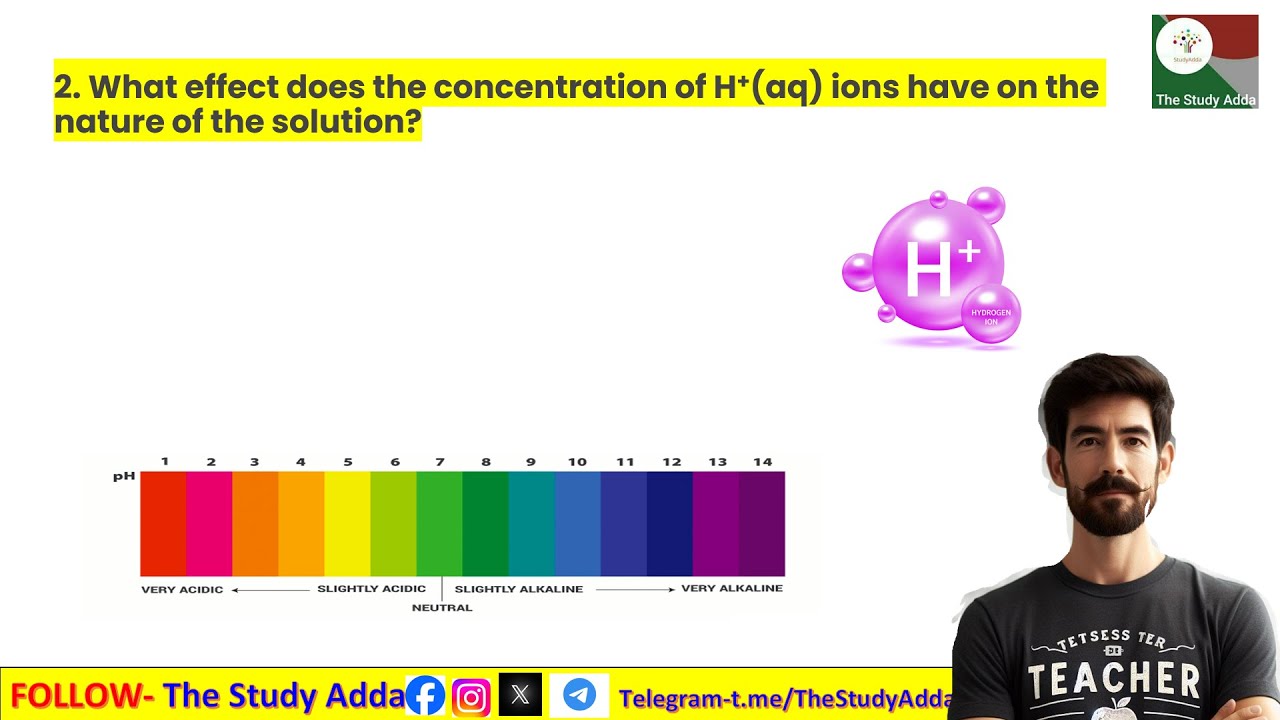
2. What effect does the concentration of H+(aq) ions have on the nature of the solution? YouTube
How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. typically, chemists write the acidic ion in solution as simply h+. Hcl h c l only. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. However, in water, h+ ions cannot exist. In solution, meaning water, a lone. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron.
From slideplayer.com
CHEMISTRY RESOURCE 9C ACIDS, BASES and SALTS ppt download How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. typically, chemists write the acidic ion in solution as simply h+. However, in water, h+ ions cannot exist. Hcl h. How Do H+ Ions Exist In Nature.
From slideplayer.com
Chapter 15 Acids and Bases ppt download How Do H+ Ions Exist In Nature In solution, meaning water, a lone. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. Hcl h c l only. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. typically, chemists write the acidic ion in solution as simply h+. However, in. How Do H+ Ions Exist In Nature.
From www.youtube.com
2. What effect does the concentration of H+(aq) ions have on the nature of the solution? YouTube How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. In solution, meaning water, a lone. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. Hcl h c l only. typically, chemists write the acidic ion in solution as simply h+. when. How Do H+ Ions Exist In Nature.
From slideplayer.com
The Chemistry and Energy of Life ppt download How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. Hcl h c l only. typically, chemists write the acidic ion in solution as simply h+. However, in water, h+. How Do H+ Ions Exist In Nature.
From byjus.com
Does H+ ion has highest mobility and thus highest conductance as compared to other ions in How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. In solution, meaning water, a lone. Hcl h c l only. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+. as h + ions dissociate. How Do H+ Ions Exist In Nature.
From www.youtube.com
H+ Electron Configuration (Hydrogen Ion) YouTube How Do H+ Ions Exist In Nature However, in water, h+ ions cannot exist. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. typically, chemists write the acidic ion in solution as simply h+. In solution, meaning water, a lone. as h + ions dissociate from the acid and bond. How Do H+ Ions Exist In Nature.
From techiescientist.com
HCl Intermolecular Forces — Type, Strong or Weak? Techiescientist How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. typically, chemists write the acidic ion in solution as simply h+. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. when hcl h c l dissolves, the proton is transferred to a. How Do H+ Ions Exist In Nature.
From www.numerade.com
Deduce the concentration of hydrogen ions (H+) and hydroxide ions (OH) for a given pH and How Do H+ Ions Exist In Nature as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. typically, chemists write the acidic ion in solution as simply h+. Hcl h c l only. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x. How Do H+ Ions Exist In Nature.
From slideplayer.com
Acids and Bases an Introduction ppt download How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. Hcl h c l only. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o. How Do H+ Ions Exist In Nature.
From www.youtube.com
what effect does concentration of H+ ions have on nature of solution acids, bases and salts How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. However, in water, h+ ions cannot exist. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. Hcl h c l only. In. How Do H+ Ions Exist In Nature.
From animalia-life.club
Hydroxide Ion How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. typically, chemists write the acidic ion in solution as simply h+. as h. How Do H+ Ions Exist In Nature.
From byjus.com
When an acid produces H+ ions in solution does that mean that they lose their acidity?? Is the How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+. Hcl h c l only. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3. How Do H+ Ions Exist In Nature.
From slideplayer.com
What Happens to an Acid or a Base in a Water Solution? ppt download How Do H+ Ions Exist In Nature In solution, meaning water, a lone. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. However, in water, h+ ions cannot exist. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. typically, chemists write the acidic ion in solution as simply h+.. How Do H+ Ions Exist In Nature.
From slideplayer.com
Introduction to Chemistry ppt download How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. In solution, meaning water, a lone. However, in water, h+ ions cannot exist. when hcl h c l dissolves, the proton is transferred to. How Do H+ Ions Exist In Nature.
From www.teachoo.com
Do basic solutions also have H+(aq) ions? If yes, then Teachoo How Do H+ Ions Exist In Nature However, in water, h+ ions cannot exist. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. In solution, meaning water, a lone. as h + ions dissociate from the. How Do H+ Ions Exist In Nature.
From slideplayer.com
How to Use This Presentation ppt download How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. In solution, meaning water, a lone. Hcl h c l only. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. However, in water, h+ ions. How Do H+ Ions Exist In Nature.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. typically, chemists write the acidic ion in solution as simply h+. In solution, meaning water, a lone. as h. How Do H+ Ions Exist In Nature.
From www.chemistrylearner.com
Iondipole Forces (Interaction) Definition and Examples How Do H+ Ions Exist In Nature However, in water, h+ ions cannot exist. In solution, meaning water, a lone. Hcl h c l only. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus. How Do H+ Ions Exist In Nature.
From www.youtube.com
How are ions formed? YouTube How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. However, in water, h+ ions cannot exist. Hcl h c l only. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. hydrogen ion, strictly,. How Do H+ Ions Exist In Nature.
From slideplayer.com
Biology, 9th ed,Sylvia Mader ppt download How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+. In solution, meaning water, a lone. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the.. How Do H+ Ions Exist In Nature.
From www.agric.wa.gov.au
Causes of soil acidity Agriculture and Food How Do H+ Ions Exist In Nature In solution, meaning water, a lone. Hcl h c l only. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. However, in water, h+ ions cannot exist. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o. How Do H+ Ions Exist In Nature.
From slideplayer.com
Acids and Bases RNA uses aminoacids to build proteins/enzymes ppt download How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. However, in water, h+ ions cannot exist. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3. How Do H+ Ions Exist In Nature.
From www.youtube.com
SES 401 Ion Exchange and Soil pH by Abdul Wakeel YouTube How Do H+ Ions Exist In Nature as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule. How Do H+ Ions Exist In Nature.
From slidetodoc.com
Where is energy stored in biomolecules like sugars How Do H+ Ions Exist In Nature as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. typically, chemists write the acidic ion in solution as simply h+. In solution, meaning water, a lone. However, in water, h+ ions cannot exist. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron.. How Do H+ Ions Exist In Nature.
From www.nagwa.com
Lesson Video Ions Nagwa How Do H+ Ions Exist In Nature as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. Hcl h c l only. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. However, in water, h+ ions cannot exist. when hcl h c l dissolves, the proton is transferred to a. How Do H+ Ions Exist In Nature.
From karsyn-blogreese.blogspot.com
Describe the Relationship Between Hydrogen Ions and Ph How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. Hcl h c l only. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from. How Do H+ Ions Exist In Nature.
From socratic.org
What is the chemical equation for HCl dissolving into water and ionizing? Socratic How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. Hcl h c l only. However, in water, h+. How Do H+ Ions Exist In Nature.
From slideplayer.com
ACIDS & BASES E. Schnobrich. ppt download How Do H+ Ions Exist In Nature Hcl h c l only. However, in water, h+ ions cannot exist. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. typically, chemists write the acidic ion in solution. How Do H+ Ions Exist In Nature.
From chemistrycommunity.nature.com
Visualizing the hydrated ions with atomic resolution Nature Research Chemistry Community How Do H+ Ions Exist In Nature typically, chemists write the acidic ion in solution as simply h+. Hcl h c l only. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its. How Do H+ Ions Exist In Nature.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. typically, chemists write the acidic ion in solution as simply h+. However, in water, h+. How Do H+ Ions Exist In Nature.
From www.youtube.com
What effect does the concentration of H+ (aq) ions have on the nature of the solution ? YouTube How Do H+ Ions Exist In Nature In solution, meaning water, a lone. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. However, in water, h+ ions cannot exist. typically, chemists write the acidic ion in solution as simply h+.. How Do H+ Ions Exist In Nature.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download ID663487 How Do H+ Ions Exist In Nature when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. Hcl. How Do H+ Ions Exist In Nature.
From www.slideserve.com
PPT Chapter 22 Properties of Water PowerPoint Presentation, free download ID6395349 How Do H+ Ions Exist In Nature Hcl h c l only. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. as h + ions dissociate from the acid and bond with water, they form hydronium. How Do H+ Ions Exist In Nature.
From slideplayer.com
Acids, Bases, & Chemical Reactions ppt download How Do H+ Ions Exist In Nature However, in water, h+ ions cannot exist. as h + ions dissociate from the acid and bond with water, they form hydronium ions, thus increasing the. In solution, meaning water, a lone. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. Hcl h c. How Do H+ Ions Exist In Nature.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download ID7021047 How Do H+ Ions Exist In Nature hydrogen ion, strictly, the nucleus of a hydrogen atom separated from its accompanying electron. In solution, meaning water, a lone. However, in water, h+ ions cannot exist. when hcl h c l dissolves, the proton is transferred to a water molecule to form hx3ox+ h x 3 o x +. as h + ions dissociate from the. How Do H+ Ions Exist In Nature.