What Is Shielding Effect In Short . It is the repulsion of valence electrons that counteracts the attraction between these. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. atomic radius generally decreases from left to right across a period of elements. this lecture is about shielding effect and screening effect in. However, with each addition of a proton there is an addition of an electron. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons.
from localrevive.com
It is the repulsion of valence electrons that counteracts the attraction between these. this lecture is about shielding effect and screening effect in. atomic radius generally decreases from left to right across a period of elements. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. However, with each addition of a proton there is an addition of an electron. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them.
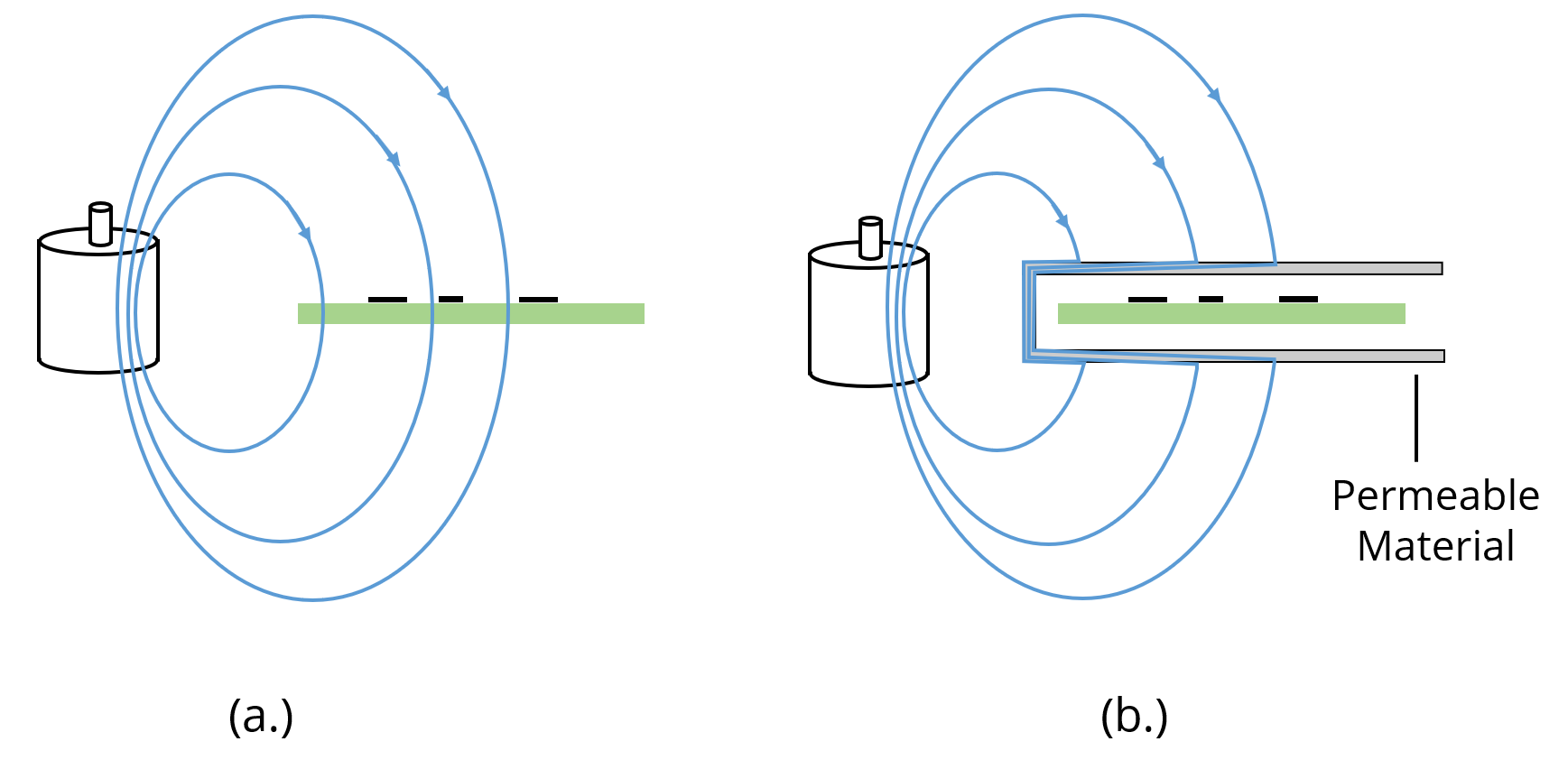
LearnEMC Introduction to Practical Shielding (2022)
What Is Shielding Effect In Short Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. However, with each addition of a proton there is an addition of an electron. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. It is the repulsion of valence electrons that counteracts the attraction between these. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. atomic radius generally decreases from left to right across a period of elements. this lecture is about shielding effect and screening effect in.
From www.youtube.com
What is Shielding Effect? shielding Effect বলতে কি বোঝ? Class 11 chemistry in bengali What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. It is the repulsion of valence electrons that counteracts the attraction between these. atomic radius generally decreases from left to right across a period of elements. However, with each addition of a proton there is an addition of. What Is Shielding Effect In Short.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry What Is Shielding Effect In Short It is the repulsion of valence electrons that counteracts the attraction between these. this lecture is about shielding effect and screening effect in. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. atomic radius generally decreases from left to right across a period of elements. the shielding effect describes. What Is Shielding Effect In Short.
From www.aakash.ac.in
What is Shielding Effect and Screening Effect? What Is Shielding Effect In Short As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. this lecture is about shielding effect and screening effect in. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. Hence, the nucleus has less grip on the outer electrons insofar. What Is Shielding Effect In Short.
From sciencestruck.com
Shielding Effect Science Struck What Is Shielding Effect In Short It is the repulsion of valence electrons that counteracts the attraction between these. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. However, with each addition of a proton there is an addition of an. What Is Shielding Effect In Short.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free download ID1158727 What Is Shielding Effect In Short Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. this lecture is about shielding effect and screening effect in. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. As the nuclear charge (z) increases, the effective nuclear charge (z*). What Is Shielding Effect In Short.
From exowuasqn.blob.core.windows.net
What Is Shielding Effect For Class 9 at Loretta Bryan blog What Is Shielding Effect In Short As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. this lecture is about shielding effect and screening effect in. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. However, with each addition of a proton there is an. What Is Shielding Effect In Short.
From www.youtube.com
What is Shielding Effect? 9th Chemistry Ch 3 Online Learning With AZ YouTube What Is Shielding Effect In Short As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. However, with each addition. What Is Shielding Effect In Short.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry What Is Shielding Effect In Short higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Shielding refers to the core. What Is Shielding Effect In Short.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID6734591 What Is Shielding Effect In Short As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. this lecture is about. What Is Shielding Effect In Short.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Engineering Projects What Is Shielding Effect In Short this lecture is about shielding effect and screening effect in. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. atomic radius generally decreases from left to right across. What Is Shielding Effect In Short.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting, and 5 Reliable Applications What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. However, with each addition of a proton there is an addition of an electron. It is the repulsion of valence electrons that counteracts the attraction between these. As the nuclear charge (z) increases, the effective nuclear charge (z*) of. What Is Shielding Effect In Short.
From thechemistrynotes.com
Shielding effect What Is Shielding Effect In Short atomic radius generally decreases from left to right across a period of elements. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. It is the repulsion of valence electrons that counteracts the attraction between these. higher levels of shielding result in a reduced attraction between the. What Is Shielding Effect In Short.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Shielding Effect In Short It is the repulsion of valence electrons that counteracts the attraction between these. this lecture is about shielding effect and screening effect in. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. However, with each addition of a proton there is an addition of an electron. . What Is Shielding Effect In Short.
From localrevive.com
LearnEMC Introduction to Practical Shielding (2022) What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases.. What Is Shielding Effect In Short.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND HOW TO USE IT IN NUMERICALS What Is Shielding Effect In Short Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of.. What Is Shielding Effect In Short.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog What Is Shielding Effect In Short However, with each addition of a proton there is an addition of an electron. this lecture is about shielding effect and screening effect in. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence. What Is Shielding Effect In Short.
From www.youtube.com
what is shielding effect and it's effect in periodic table chemistryclasses612 YouTube What Is Shielding Effect In Short higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons.. What Is Shielding Effect In Short.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Is Shielding Effect In Short Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. the shielding effect. What Is Shielding Effect In Short.
From mungfali.com
What Is Shielding Effect What Is Shielding Effect In Short It is the repulsion of valence electrons that counteracts the attraction between these. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. atomic radius generally decreases from left to. What Is Shielding Effect In Short.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is Shielding Effect In Short However, with each addition of a proton there is an addition of an electron. this lecture is about shielding effect and screening effect in. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. . What Is Shielding Effect In Short.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Shielding Effect In Short However, with each addition of a proton there is an addition of an electron. atomic radius generally decreases from left to right across a period of elements. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. As the nuclear charge (z) increases, the effective nuclear charge (z*). What Is Shielding Effect In Short.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND HOW TO USE IT IN NUMERICALS What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. It is the repulsion of valence electrons that counteracts the attraction between these. atomic radius generally decreases from left to right across a period of elements. Shielding refers to the core electrons repelling the outer electrons, which lowers. What Is Shielding Effect In Short.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog What Is Shielding Effect In Short Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. It is the repulsion of valence electrons that counteracts the attraction between these. the shielding effect describes how electrons closer to the nucleus shield the. What Is Shielding Effect In Short.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND HOW TO USE IT IN NUMERICALS What Is Shielding Effect In Short Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. atomic radius generally decreases from left to right across a period of elements. As the nuclear. What Is Shielding Effect In Short.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is Shielding Effect In Short Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. atomic radius generally decreases from left to right across a period of elements. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. this lecture is about shielding effect and. What Is Shielding Effect In Short.
From zandex.org
What is shielding effect with example? Zandex What Is Shielding Effect In Short atomic radius generally decreases from left to right across a period of elements. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. It is the repulsion of valence electrons that. What Is Shielding Effect In Short.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. It is the repulsion of valence electrons that counteracts the attraction between these. Hence, the nucleus has less grip on the outer. What Is Shielding Effect In Short.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect In Short the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. this lecture is about shielding effect and screening effect in. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. As the nuclear charge (z) increases, the effective nuclear charge (z*). What Is Shielding Effect In Short.
From exowuasqn.blob.core.windows.net
What Is Shielding Effect For Class 9 at Loretta Bryan blog What Is Shielding Effect In Short this lecture is about shielding effect and screening effect in. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. It is the repulsion of valence electrons that counteracts the attraction between these. However, with each addition of a proton there is an addition of an electron.. What Is Shielding Effect In Short.
From www.youtube.com
What is Shielding Effect in Simple Words Shielding Effect Definition, Examples , Trend YouTube What Is Shielding Effect In Short this lecture is about shielding effect and screening effect in. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of. atomic radius generally decreases from left to right across a period of elements. It is the repulsion of valence electrons that counteracts the attraction between these. . What Is Shielding Effect In Short.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE What Is Shielding Effect In Short atomic radius generally decreases from left to right across a period of elements. It is the repulsion of valence electrons that counteracts the attraction between these. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. this lecture is about shielding effect and screening effect in. . What Is Shielding Effect In Short.
From www.youtube.com
Shielding Effect Lecture 07 Chapter 03 Chemistry Class 9th YouTube What Is Shielding Effect In Short It is the repulsion of valence electrons that counteracts the attraction between these. higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic. atomic radius generally decreases from left to right across a period of elements. Hence, the nucleus has less grip on the outer electrons insofar as. What Is Shielding Effect In Short.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog What Is Shielding Effect In Short Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. It is the repulsion of valence electrons that counteracts the attraction between these. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. higher levels of shielding result in a. What Is Shielding Effect In Short.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog What Is Shielding Effect In Short this lecture is about shielding effect and screening effect in. As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Hence, the nucleus has less grip on the outer electrons insofar as it is shielded from them. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge. What Is Shielding Effect In Short.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry What Is Shielding Effect In Short As the nuclear charge (z) increases, the effective nuclear charge (z*) of the valence electrons also increases. Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. the shielding effect describes how electrons closer to the nucleus shield the electrons farther away from the positive charge of.. What Is Shielding Effect In Short.