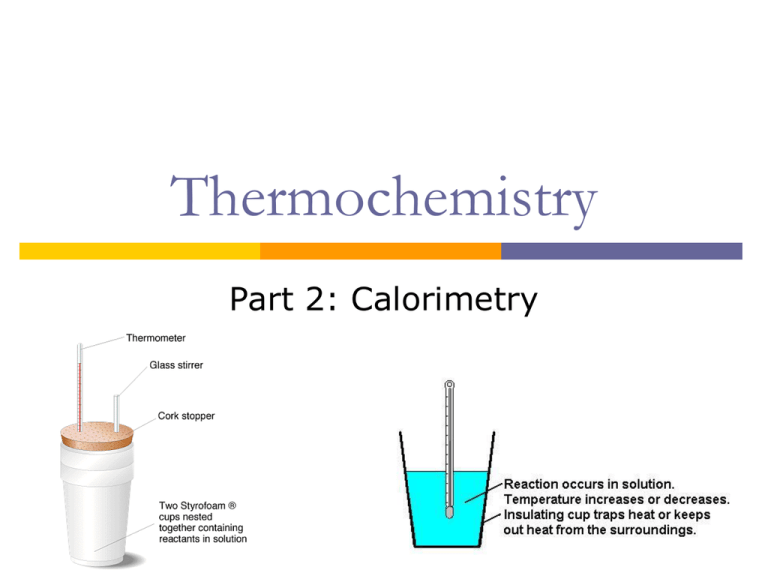
What Is Q In Calorimetry . When heat is absorbed by the solution, q for the solution has a positive value. Q calorimeter represents the calorimeter and everything in it. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q = measure of heat transfer. C = specific heat of the body. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. In calorimetry, q represents the heat absorbed or released during a chemical reaction. Δt = change in the temperature. It is calculated using the formula q = mcδt, where. M = mass of the body. Q is given a positive (+) sign. That is, all the heat exchanged with the reaction is absorbed or.
from studylib.net
That is, all the heat exchanged with the reaction is absorbed or. C = specific heat of the body. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q is given a positive (+) sign. Q = measure of heat transfer. When heat is absorbed by the solution, q for the solution has a positive value. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. It is calculated using the formula q = mcδt, where. In calorimetry, q represents the heat absorbed or released during a chemical reaction. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter.
Calorimetry
What Is Q In Calorimetry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. It is calculated using the formula q = mcδt, where. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. When heat is absorbed by the solution, q for the solution has a positive value. In calorimetry, q represents the heat absorbed or released during a chemical reaction. M = mass of the body. Q is given a positive (+) sign. Q = measure of heat transfer. Δt = change in the temperature. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. That is, all the heat exchanged with the reaction is absorbed or. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. C = specific heat of the body. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q calorimeter represents the calorimeter and everything in it.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Is Q In Calorimetry In calorimetry, q represents the heat absorbed or released during a chemical reaction. Q calorimeter represents the calorimeter and everything in it. C = specific heat of the body. M = mass of the body. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In a calorimetric determination, either (a) an exothermic process occurs. What Is Q In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry What Is Q In Calorimetry Q = measure of heat transfer. In calorimetry, q represents the heat absorbed or released during a chemical reaction. When heat is absorbed by the solution, q for the solution has a positive value. Δt = change in the temperature. C = specific heat of the body. Q is given a positive (+) sign. Q calorimeter represents the calorimeter and. What Is Q In Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Q In Calorimetry This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. When heat is absorbed by the solution, q for the solution has a positive value. Δt = change in the temperature. That is, all the heat exchanged with the reaction is absorbed or. Q calorimeter represents the calorimeter and everything in. What Is Q In Calorimetry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry What Is Q In Calorimetry That is, all the heat exchanged with the reaction is absorbed or. When heat is absorbed by the solution, q for the solution has a positive value. Q is given a positive (+) sign. C = specific heat of the body. In calorimetry, q represents the heat absorbed or released during a chemical reaction. The formula q = ccal ×. What Is Q In Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is Q In Calorimetry Q is given a positive (+) sign. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Q = measure of heat transfer. Q calorimeter represents the calorimeter and everything in it. It is calculated using the formula q = mcδt, where. That is, all the heat exchanged with the reaction is absorbed or. In. What Is Q In Calorimetry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is Q In Calorimetry M = mass of the body. That is, all the heat exchanged with the reaction is absorbed or. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where. What Is Q In Calorimetry.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube What Is Q In Calorimetry M = mass of the body. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. That is, all the heat exchanged with the reaction is absorbed or. C = specific heat of the body. In calorimetry, q represents the heat absorbed or released during a chemical reaction. Q calorimeter represents the calorimeter and everything. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 What Is Q In Calorimetry Q = measure of heat transfer. Q calorimeter represents the calorimeter and everything in it. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Q is given a positive (+) sign. M = mass of the body. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that. What Is Q In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube What Is Q In Calorimetry In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Q = measure of heat transfer. That is, all the heat exchanged with the reaction is absorbed or. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Q In Calorimetry C = specific heat of the body. In calorimetry, q represents the heat absorbed or released during a chemical reaction. That is, all the heat exchanged with the reaction is absorbed or. Δt = change in the temperature. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. Calorimetry measures enthalpy. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Is Q In Calorimetry In calorimetry, q represents the heat absorbed or released during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. M = mass of the body. Δt = change in the temperature. When heat is absorbed by the solution, q for the solution has a positive value. Q = measure of heat. What Is Q In Calorimetry.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow What Is Q In Calorimetry The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. C = specific heat of the body. Δt = change in the temperature. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Q is. What Is Q In Calorimetry.
From studylib.net
PPT Calorimetry What Is Q In Calorimetry Q is given a positive (+) sign. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. M = mass of the body. That is, all the heat exchanged with the reaction is absorbed or. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. What Is Q In Calorimetry.
From saylordotorg.github.io
Calorimetry What Is Q In Calorimetry M = mass of the body. C = specific heat of the body. Q = measure of heat transfer. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. When heat is absorbed by the solution, q for the solution has a positive value. Δt = change in the temperature. In calorimetry, q represents the. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Q In Calorimetry This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. C = specific heat of the body. When heat is absorbed by the solution, q for the solution has a positive value. Δt = change in the temperature. In calorimetry, q represents the heat absorbed or released during a chemical reaction.. What Is Q In Calorimetry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is Q In Calorimetry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Δt = change in the temperature. C = specific heat of the body. In calorimetry, q represents the heat absorbed or released during a chemical reaction. Q = measure of heat transfer. When heat is absorbed by the solution, q for the solution has a. What Is Q In Calorimetry.
From users.highland.edu
Calorimetry What Is Q In Calorimetry That is, all the heat exchanged with the reaction is absorbed or. M = mass of the body. Δt = change in the temperature. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. Q = measure of heat transfer. C = specific heat of the body. In a calorimetric determination, either (a) an exothermic. What Is Q In Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube What Is Q In Calorimetry Q calorimeter represents the calorimeter and everything in it. In calorimetry, q represents the heat absorbed or released during a chemical reaction. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. It is calculated using the formula q = mcδt, where. Q is given a positive (+) sign. M =. What Is Q In Calorimetry.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 What Is Q In Calorimetry It is calculated using the formula q = mcδt, where. Δt = change in the temperature. The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. C = specific heat of the body. Q is given a positive (+) sign. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends.. What Is Q In Calorimetry.
From studylib.net
Heat Equation What Is Q In Calorimetry C = specific heat of the body. Δt = change in the temperature. When heat is absorbed by the solution, q for the solution has a positive value. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. This means. What Is Q In Calorimetry.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter What Is Q In Calorimetry M = mass of the body. Q calorimeter represents the calorimeter and everything in it. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. This means that the reaction produces heat for the solution to absorb and q for. What Is Q In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry What Is Q In Calorimetry In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Q = measure of heat transfer. That is, all the heat exchanged with the reaction is absorbed or. It is calculated using the formula q = mcδt, where. Δt =. What Is Q In Calorimetry.
From studylib.net
Calorimetry What Is Q In Calorimetry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In calorimetry, q represents the heat absorbed or released during a chemical reaction. That is, all the heat exchanged with the reaction is absorbed or. Q = measure of heat transfer. When heat is absorbed by the solution, q for the solution has a positive. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Q In Calorimetry When heat is absorbed by the solution, q for the solution has a positive value. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an.. What Is Q In Calorimetry.
From www.science-revision.co.uk
Calorimetry What Is Q In Calorimetry It is calculated using the formula q = mcδt, where. In calorimetry, q represents the heat absorbed or released during a chemical reaction. Δt = change in the temperature. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. C = specific heat of the body. Q is given a positive. What Is Q In Calorimetry.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Q In Calorimetry Q = measure of heat transfer. M = mass of the body. Q is given a positive (+) sign. Δt = change in the temperature. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is. What Is Q In Calorimetry.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to What Is Q In Calorimetry Q is given a positive (+) sign. When heat is absorbed by the solution, q for the solution has a positive value. Δt = change in the temperature. That is, all the heat exchanged with the reaction is absorbed or. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. Calorimetry. What Is Q In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry I What Is Q In Calorimetry Q is given a positive (+) sign. In a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its surroundings, or (b) an. Q calorimeter represents the calorimeter and everything in it. C = specific heat of the body. In calorimetry, q represents the heat absorbed. What Is Q In Calorimetry.
From studylib.net
Calorimetry What Is Q In Calorimetry When heat is absorbed by the solution, q for the solution has a positive value. Q is given a positive (+) sign. Δt = change in the temperature. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q = measure of heat transfer. The formula q = ccal × δt is essential for calorimetry. What Is Q In Calorimetry.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow What Is Q In Calorimetry The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. It is calculated using the formula q = mcδt, where. Q is given a positive (+) sign. In calorimetry, q represents the heat absorbed or released during a chemical reaction. M = mass of the body. Q calorimeter represents the calorimeter and everything in it.. What Is Q In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 What Is Q In Calorimetry That is, all the heat exchanged with the reaction is absorbed or. It is calculated using the formula q = mcδt, where. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q calorimeter represents the calorimeter and everything in it. In calorimetry, q represents the heat absorbed or released during a chemical reaction. In. What Is Q In Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent What Is Q In Calorimetry Q calorimeter represents the calorimeter and everything in it. Q is given a positive (+) sign. It is calculated using the formula q = mcδt, where. Q = measure of heat transfer. Δt = change in the temperature. In calorimetry, q represents the heat absorbed or released during a chemical reaction. In a calorimetric determination, either (a) an exothermic process. What Is Q In Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent What Is Q In Calorimetry The formula q = ccal × δt is essential for calorimetry experiments using a calorimeter. That is, all the heat exchanged with the reaction is absorbed or. It is calculated using the formula q = mcδt, where. M = mass of the body. C = specific heat of the body. Δt = change in the temperature. This means that the. What Is Q In Calorimetry.
From www.tes.com
POWER POINT CALORIMETRY Power Point Q = MCT Grade 12 Chemistry Power What Is Q In Calorimetry Q = measure of heat transfer. When heat is absorbed by the solution, q for the solution has a positive value. M = mass of the body. Δt = change in the temperature. Q calorimeter represents the calorimeter and everything in it. C = specific heat of the body. That is, all the heat exchanged with the reaction is absorbed. What Is Q In Calorimetry.
From studylib.net
Calorimetry What Is Q In Calorimetry That is, all the heat exchanged with the reaction is absorbed or. Q is given a positive (+) sign. Δt = change in the temperature. In calorimetry, q represents the heat absorbed or released during a chemical reaction. It is calculated using the formula q = mcδt, where. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. What Is Q In Calorimetry.