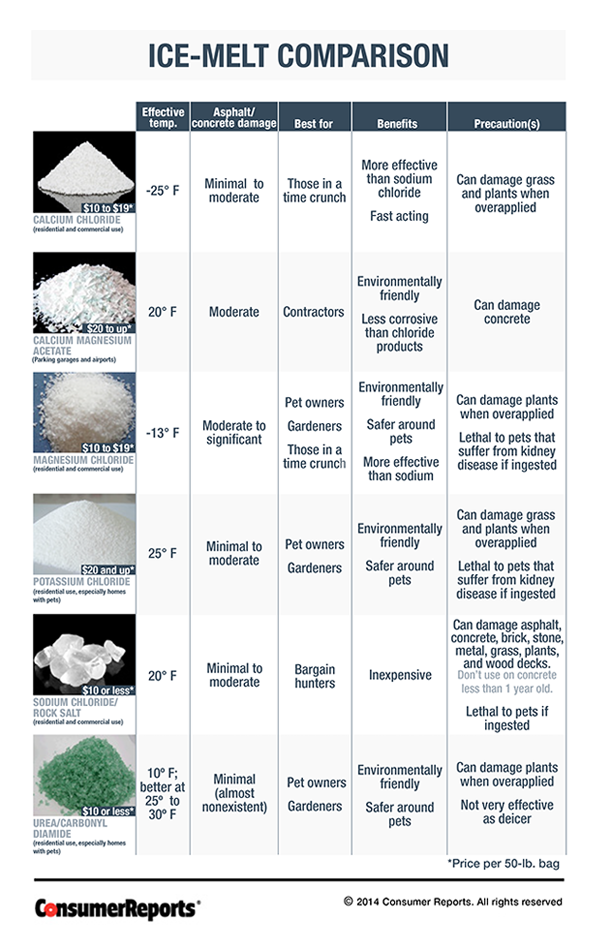
Rock Salt Melt Ice Temperature . The more surface area salt can cover, the better the chances for melting ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Water freezes at 32 degrees fahrenheit; For example, calcium chloride lowers the freezing point more than sodium chloride. But adding salt plummets that freezing point down. Yes, it can be too cold for salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. The working temperature range isn't the same for all types of salt. This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. Melting is endothermic, so it lowers the temperature.
from www.consumerreports.org
Yes, it can be too cold for salt to melt ice. Melting is endothermic, so it lowers the temperature. When you mix salt onto that layer, it slowly lowers its melting point. The working temperature range isn't the same for all types of salt. For example, calcium chloride lowers the freezing point more than sodium chloride. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. But adding salt plummets that freezing point down. Salt makes ice colder because the salt prevents melted water from freezing. Water freezes at 32 degrees fahrenheit; This phenomenon is called freezing point depression.
Best Rock Salt and Ice Melts Review Consumer Reports
Rock Salt Melt Ice Temperature The working temperature range isn't the same for all types of salt. Salt makes ice colder because the salt prevents melted water from freezing. The more surface area salt can cover, the better the chances for melting ice. Water freezes at 32 degrees fahrenheit; Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium chloride. Yes, it can be too cold for salt to melt ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The working temperature range isn't the same for all types of salt.
From www.consumerreports.org
Best Rock Salt and Ice Melts Review Consumer Reports Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. Salt makes ice colder because the salt prevents melted water from freezing. Yes, it can be too cold for salt to melt ice. The working temperature range isn't the same for all types of salt. When you mix salt onto that layer, it slowly lowers its melting point.. Rock Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Rock Salt Melt Ice Temperature Salt makes ice colder because the salt prevents melted water from freezing. Yes, it can be too cold for salt to melt ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. This phenomenon is called freezing point depression. When you mix salt onto that layer,. Rock Salt Melt Ice Temperature.
From www.lowes.com
Hot Rock 50 lbs. Rock Salt Ice Melt in the Ice Melt department at Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The more surface area salt can cover, the better the chances for melting ice. Water freezes at 32 degrees fahrenheit; Melting is endothermic, so it lowers the temperature. For example, calcium chloride lowers the freezing point more. Rock Salt Melt Ice Temperature.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. For example, calcium chloride lowers the freezing point more than sodium chloride. The more surface area salt can cover, the better the chances for melting ice. Salt makes ice colder because the salt prevents melted water from. Rock Salt Melt Ice Temperature.
From www.lowes.com
SafeStep 50lb Natural Nacl Rock Salt Ice Melt Salt in the Ice Melt Rock Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Melting is endothermic, so it lowers the temperature. This phenomenon is called freezing point depression. Salt makes ice colder because the salt prevents. Rock Salt Melt Ice Temperature.
From ninjadeicer.com
Rock Salt vs Ice Melt What’s the Difference? Ninja DeIcer Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Water freezes at 32 degrees fahrenheit; But adding salt plummets that freezing point down. Yes, it can be. Rock Salt Melt Ice Temperature.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Rock Salt Melt Ice Temperature But adding salt plummets that freezing point down. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Salt makes ice colder because the salt prevents melted water from freezing. Melting is endothermic, so it lowers the temperature. When you mix salt onto that layer, it slowly. Rock Salt Melt Ice Temperature.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. Water freezes at 32 degrees fahrenheit; Yes, it can be too cold for salt to melt ice. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. Salt makes ice colder. Rock Salt Melt Ice Temperature.
From www.homedepot.com
Snow Joe Melt 50 lbs. Sodium Rock Salt Ice Melt MELT50RS The Home Depot Rock Salt Melt Ice Temperature Yes, it can be too cold for salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. When you mix salt onto that layer, it slowly lowers its melting point. The working temperature range isn't the same for all types of salt. But adding salt plummets that freezing point down. For example, calcium chloride. Rock Salt Melt Ice Temperature.
From greengobbler.com
Difference Between Rock Salt and Ice Melt Rock Salt Melt Ice Temperature Yes, it can be too cold for salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better the chances for melting ice. But adding salt plummets that freezing point down. The key. Rock Salt Melt Ice Temperature.
From greengobbler.com
Difference Between Rock Salt and Ice Melt Rock Salt Melt Ice Temperature When you mix salt onto that layer, it slowly lowers its melting point. The working temperature range isn't the same for all types of salt. The more surface area salt can cover, the better the chances for melting ice. For example, calcium chloride lowers the freezing point more than sodium chloride. This phenomenon is called freezing point depression. Melting is. Rock Salt Melt Ice Temperature.
From www.alamy.com
macro above view of a single rock salt icemelt on concrete with a Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Water freezes at 32 degrees fahrenheit; Salt makes ice colder because the salt prevents melted water from freezing. But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium. Rock Salt Melt Ice Temperature.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Rock Salt Melt Ice Temperature Melting is endothermic, so it lowers the temperature. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Yes, it can be too cold for salt to melt ice. When you mix salt onto that layer, it slowly lowers its melting point. The more surface area salt can cover, the better. Rock Salt Melt Ice Temperature.
From sciencing.com
Rock Salt Vs. Table Salt to Melt Ice Sciencing Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. Yes, it can be too cold for salt to melt ice. The working temperature range isn't the same for all types of salt. Salt makes ice colder because the salt prevents melted water from freezing. The more surface area salt can cover, the better the chances for melting. Rock Salt Melt Ice Temperature.
From tennier.ca
Melting the ice What you need to know about Rock Salt vs Ice Melt Rock Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. Salt makes ice colder because the salt prevents melted water from freezing. But adding salt plummets that freezing point down. The working temperature range isn't the same for all types of salt. Melting is endothermic, so it lowers the temperature. When you mix salt onto that. Rock Salt Melt Ice Temperature.
From meltsnow.com
Salt vs. Temperature Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The working temperature range isn't the same for all types of salt. But adding salt plummets that freezing point down. Salt makes ice colder because the salt prevents melted water from freezing. The more surface area salt. Rock Salt Melt Ice Temperature.
From howgem.com
How Long Does It Take for Ice To Melt at Average Room Temperature Rock Salt Melt Ice Temperature Water freezes at 32 degrees fahrenheit; This phenomenon is called freezing point depression. Melting is endothermic, so it lowers the temperature. The working temperature range isn't the same for all types of salt. But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium chloride. When you mix salt onto that layer,. Rock Salt Melt Ice Temperature.
From www.themunicipal.com
Minimizing the impact of ice The Municipal Rock Salt Melt Ice Temperature The working temperature range isn't the same for all types of salt. The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. Yes, it can be too cold for salt to melt ice. Water freezes at 32 degrees fahrenheit; But adding salt plummets. Rock Salt Melt Ice Temperature.
From www.merchantsgroup.com
Rock Salt vs. Ice Melt and How to Use Them Correctly Rock Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The working temperature range isn't the same for all types of salt. For example, calcium chloride lowers the freezing point more than sodium. Rock Salt Melt Ice Temperature.
From safepaw.com
What temperature does salt and ice melt work? Safe Paw Rock Salt Melt Ice Temperature Yes, it can be too cold for salt to melt ice. Water freezes at 32 degrees fahrenheit; The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium chloride.. Rock Salt Melt Ice Temperature.
From greengobbler.com
The Importance of Using a Safe Ice Melt Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. Melting is endothermic, so it lowers the temperature. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. This phenomenon is called freezing point depression. But adding salt plummets that freezing point down. Salt makes. Rock Salt Melt Ice Temperature.
From shawnacoronado.com
Ice Melt Versus Rock Salt in the Garden Shawna Coronado Rock Salt Melt Ice Temperature The working temperature range isn't the same for all types of salt. Yes, it can be too cold for salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. Water freezes at 32 degrees fahrenheit; When you mix salt onto that layer, it slowly lowers its melting point. But adding salt plummets that freezing. Rock Salt Melt Ice Temperature.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Rock Salt Melt Ice Temperature Yes, it can be too cold for salt to melt ice. Salt makes ice colder because the salt prevents melted water from freezing. When you mix salt onto that layer, it slowly lowers its melting point. But adding salt plummets that freezing point down. Water freezes at 32 degrees fahrenheit; The working temperature range isn't the same for all types. Rock Salt Melt Ice Temperature.
From ninjadeicer.com
Rock Salt vs Ice Melt What’s the Difference? Ninja DeIcer Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. Yes, it can be too cold for salt to melt ice. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. The working temperature range isn't the same for all types of salt. But adding. Rock Salt Melt Ice Temperature.
From lessonberginmoabites.z21.web.core.windows.net
How Does Salt Interact With Ice Rock Salt Melt Ice Temperature But adding salt plummets that freezing point down. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Salt makes ice colder because the salt prevents melted water from freezing. The working temperature range isn't the same for all types of salt. Yes, it can be too. Rock Salt Melt Ice Temperature.
From www.cleanlink.com
Optimal Ice Melt Types, Application Strategies Rock Salt Melt Ice Temperature Water freezes at 32 degrees fahrenheit; When you mix salt onto that layer, it slowly lowers its melting point. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Melting is endothermic, so it lowers the temperature. For example, calcium chloride lowers the freezing point more than. Rock Salt Melt Ice Temperature.
From northrockmineral.com
Ice Melt vs. Rock Salt Snow Joe, LLC. Rock Salt Melt Ice Temperature Melting is endothermic, so it lowers the temperature. When you mix salt onto that layer, it slowly lowers its melting point. Yes, it can be too cold for salt to melt ice. The more surface area salt can cover, the better the chances for melting ice. But adding salt plummets that freezing point down. Water freezes at 32 degrees fahrenheit;. Rock Salt Melt Ice Temperature.
From rocksaltandicecontrolhq.com
Rock Salt Melt Ice Temperature Yes, it can be too cold for salt to melt ice. This phenomenon is called freezing point depression. Water freezes at 32 degrees fahrenheit; The working temperature range isn't the same for all types of salt. Melting is endothermic, so it lowers the temperature. The key is, there has to be at least a tiny bit of water on the. Rock Salt Melt Ice Temperature.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC Rock Salt Melt Ice Temperature For example, calcium chloride lowers the freezing point more than sodium chloride. The more surface area salt can cover, the better the chances for melting ice. Water freezes at 32 degrees fahrenheit; But adding salt plummets that freezing point down. Yes, it can be too cold for salt to melt ice. Melting is endothermic, so it lowers the temperature. When. Rock Salt Melt Ice Temperature.
From www.dreamstime.com
Ice Melt Rock Salt is Being Spread on Your Walking Path To Melt the Rock Salt Melt Ice Temperature The working temperature range isn't the same for all types of salt. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. For example, calcium chloride lowers the freezing point more than sodium chloride. Melting is endothermic, so it lowers the temperature. Yes, it can be too. Rock Salt Melt Ice Temperature.
From www.bigstockphoto.com
Rock Salt Ice Melt Image & Photo (Free Trial) Bigstock Rock Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. The working temperature range isn't the same for all types of salt. Yes, it can be too cold for salt to melt ice. Melting is endothermic, so it lowers the temperature. The key is, there has to be at least a tiny bit of water on. Rock Salt Melt Ice Temperature.
From www.youtube.com
How is rock salt ice melter made? YouTube Rock Salt Melt Ice Temperature Water freezes at 32 degrees fahrenheit; But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium chloride. The working temperature range isn't the same for all types of salt. Yes, it can be too cold for salt to melt ice. Melting is endothermic, so it lowers the temperature. The more surface. Rock Salt Melt Ice Temperature.
From heidijricker.blob.core.windows.net
Is Rock Salt Used To Melt Ice at heidijricker blog Rock Salt Melt Ice Temperature The more surface area salt can cover, the better the chances for melting ice. The working temperature range isn't the same for all types of salt. But adding salt plummets that freezing point down. For example, calcium chloride lowers the freezing point more than sodium chloride. This phenomenon is called freezing point depression. When you mix salt onto that layer,. Rock Salt Melt Ice Temperature.
From quizdbcornwallis.z21.web.core.windows.net
What Temperature Does Rock Melt Rock Salt Melt Ice Temperature This phenomenon is called freezing point depression. The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Water freezes at 32 degrees fahrenheit; Melting is endothermic, so it lowers the temperature. The working temperature range isn't the same for all types of salt. For example, calcium chloride. Rock Salt Melt Ice Temperature.
From www.northeastnursery.com
Is All Ice Melt Created Equal? Northeast Nursery Rock Salt Melt Ice Temperature The key is, there has to be at least a tiny bit of water on the road for freezing point depression to work. Melting is endothermic, so it lowers the temperature. But adding salt plummets that freezing point down. Yes, it can be too cold for salt to melt ice. This phenomenon is called freezing point depression. Water freezes at. Rock Salt Melt Ice Temperature.