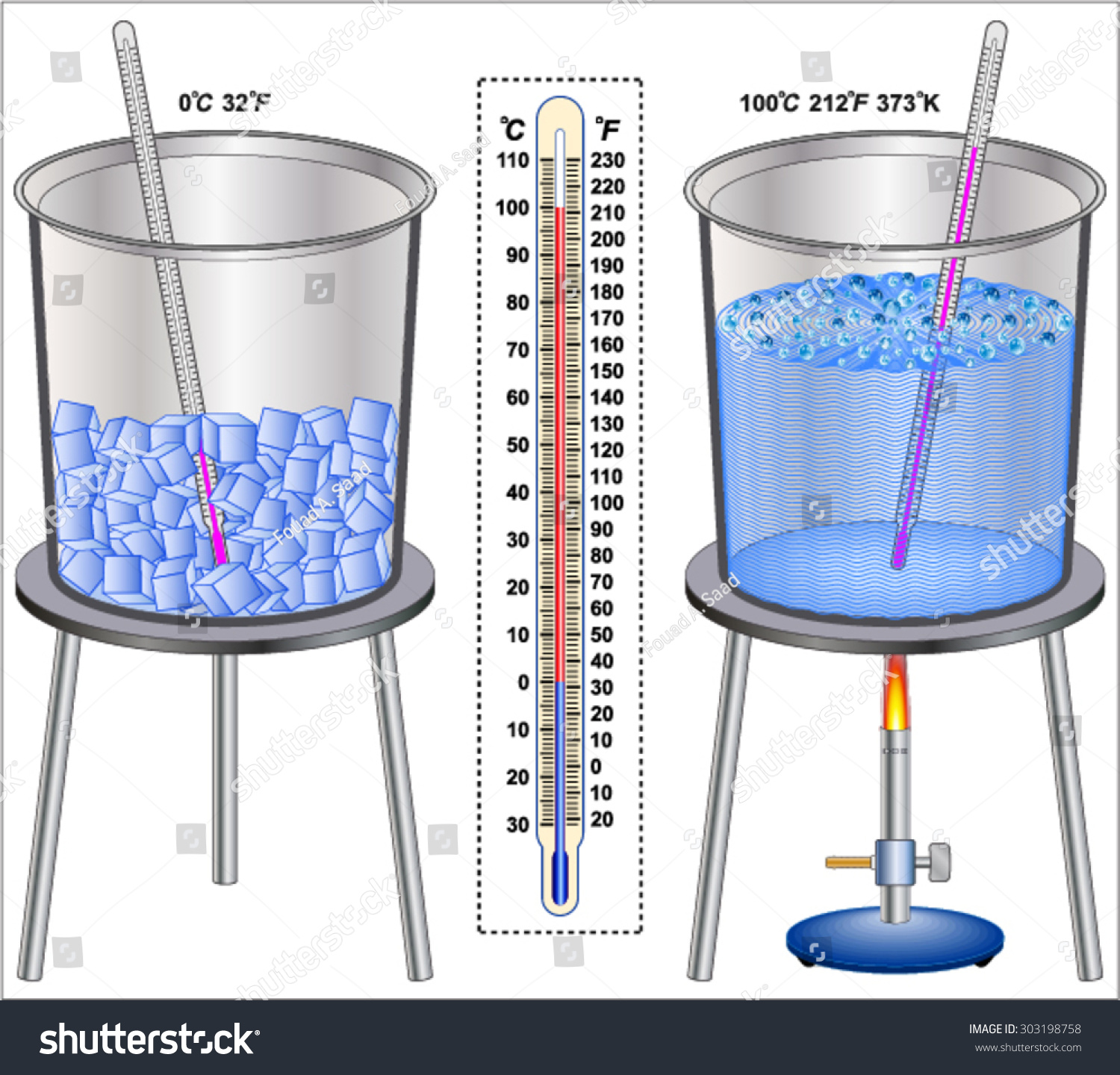
The Temperature Of Boiling Water Extensive Or Intensive . intensive properties, in contrast, do not depend on the amount of the substance; Mass and volume are examples of extensive properties. there are two conventions regarding the standard boiling point of water: physical chemist and physicist richard c. A quick test to decide whether a. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. an extensive property is a property that depends on the amount of matter in a sample. Here is an explanation of what. temperature is an intensive variable as, for example, is the density of liquids. Tolman coined the terms “intensive” and “extensive” in 1917. The normal boiling point is commonly given as 100 °c.
from dxoqzfoie.blob.core.windows.net
temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. there are two conventions regarding the standard boiling point of water: Tolman coined the terms “intensive” and “extensive” in 1917. Mass and volume are examples of extensive properties. intensive properties, in contrast, do not depend on the amount of the substance; an extensive property is a property that depends on the amount of matter in a sample. A quick test to decide whether a. Here is an explanation of what. physical chemist and physicist richard c. The normal boiling point is commonly given as 100 °c.
When Does Water Boil Temperature at Kathleen Conley blog
The Temperature Of Boiling Water Extensive Or Intensive an extensive property is a property that depends on the amount of matter in a sample. an extensive property is a property that depends on the amount of matter in a sample. intensive properties, in contrast, do not depend on the amount of the substance; there are two conventions regarding the standard boiling point of water: Tolman coined the terms “intensive” and “extensive” in 1917. The normal boiling point is commonly given as 100 °c. Here is an explanation of what. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. temperature is an intensive variable as, for example, is the density of liquids. Mass and volume are examples of extensive properties. physical chemist and physicist richard c. A quick test to decide whether a.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. Tolman coined the terms “intensive” and “extensive” in 1917. Here is an explanation of what. A quick test to decide whether a. intensive properties, in. The Temperature Of Boiling Water Extensive Or Intensive.
From studylib.net
Boiling Point = Temperature at which a liquid turns into a gas The Temperature Of Boiling Water Extensive Or Intensive an extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. there are two conventions regarding the standard boiling point of water: temperature is an intensive variable as, for example, is the density of liquids. Tolman coined the terms “intensive” and “extensive” in 1917.. The Temperature Of Boiling Water Extensive Or Intensive.
From slideplayer.com
PHYSICAL AND CHEMICAL PROPERTIES ppt download The Temperature Of Boiling Water Extensive Or Intensive intensive properties, in contrast, do not depend on the amount of the substance; Here is an explanation of what. there are two conventions regarding the standard boiling point of water: temperature is an intensive variable as, for example, is the density of liquids. The normal boiling point is commonly given as 100 °c. A quick test to. The Temperature Of Boiling Water Extensive Or Intensive.
From www.numerade.com
SOLVED Boiling Water At what temperature would 250 mL of water boil? 1000 mL ? Is the boiling The Temperature Of Boiling Water Extensive Or Intensive physical chemist and physicist richard c. The normal boiling point is commonly given as 100 °c. an extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. A quick test to decide whether a. intensive properties, in contrast, do not depend on the amount. The Temperature Of Boiling Water Extensive Or Intensive.
From www.slideshare.net
Chapter 3 Matter Properties And Changes The Temperature Of Boiling Water Extensive Or Intensive temperature is an intensive variable as, for example, is the density of liquids. The normal boiling point is commonly given as 100 °c. Mass and volume are examples of extensive properties. intensive properties, in contrast, do not depend on the amount of the substance; an extensive property is a property that depends on the amount of matter. The Temperature Of Boiling Water Extensive Or Intensive.
From dxoqzfoie.blob.core.windows.net
When Does Water Boil Temperature at Kathleen Conley blog The Temperature Of Boiling Water Extensive Or Intensive intensive properties, in contrast, do not depend on the amount of the substance; there are two conventions regarding the standard boiling point of water: Mass and volume are examples of extensive properties. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. The normal boiling point. The Temperature Of Boiling Water Extensive Or Intensive.
From www.pinterest.com
Mastering Boiling Water Tips for Cooking at Altitude The Temperature Of Boiling Water Extensive Or Intensive intensive properties, in contrast, do not depend on the amount of the substance; Tolman coined the terms “intensive” and “extensive” in 1917. there are two conventions regarding the standard boiling point of water: physical chemist and physicist richard c. Mass and volume are examples of extensive properties. A quick test to decide whether a. an extensive. The Temperature Of Boiling Water Extensive Or Intensive.
From dxopohezx.blob.core.windows.net
What Is Water S Boiling Temp at Greg Clark blog The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. The normal boiling point is commonly given as 100 °c. Here is an explanation of what. temperature is an intensive variable as, for example, is. The Temperature Of Boiling Water Extensive Or Intensive.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. Here is an explanation of what. Mass and volume are examples of extensive properties. there are two conventions regarding the standard boiling point of water: intensive properties, in contrast, do not depend on the amount of the substance; physical chemist and physicist richard c. Tolman coined the terms “intensive” and. The Temperature Of Boiling Water Extensive Or Intensive.
From www.peoi.org
Chapter 10 Section C Properties of Liquids The Temperature Of Boiling Water Extensive Or Intensive The normal boiling point is commonly given as 100 °c. an extensive property is a property that depends on the amount of matter in a sample. Tolman coined the terms “intensive” and “extensive” in 1917. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. there. The Temperature Of Boiling Water Extensive Or Intensive.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Industrial Heat The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperature is an intensive variable as, for example, is the density of liquids. Tolman coined the terms “intensive” and “extensive” in 1917. The normal boiling point is commonly given as 100 °c. intensive properties, in contrast, do not depend on the amount of the substance; Mass. The Temperature Of Boiling Water Extensive Or Intensive.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Vector Illustration of The Temperature Of Boiling Water Extensive Or Intensive Here is an explanation of what. an extensive property is a property that depends on the amount of matter in a sample. physical chemist and physicist richard c. Tolman coined the terms “intensive” and “extensive” in 1917. temperature is an intensive variable as, for example, is the density of liquids. A quick test to decide whether a.. The Temperature Of Boiling Water Extensive Or Intensive.
From www.numerade.com
SOLVEDBoiling Water At what temperature would 250 mL of water boil? 1000 mL ? Is the boiling The Temperature Of Boiling Water Extensive Or Intensive temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. an extensive property is a property that depends on the amount of matter in a sample. A quick test to decide whether a. Here is an explanation of what. temperature is an intensive variable as, for. The Temperature Of Boiling Water Extensive Or Intensive.
From www.researchgate.net
Water Boiling Test showing the temperature of 1 liter of water with... Download Scientific Diagram The Temperature Of Boiling Water Extensive Or Intensive physical chemist and physicist richard c. temperature is an intensive variable as, for example, is the density of liquids. The normal boiling point is commonly given as 100 °c. intensive properties, in contrast, do not depend on the amount of the substance; Mass and volume are examples of extensive properties. there are two conventions regarding the. The Temperature Of Boiling Water Extensive Or Intensive.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful The Temperature Of Boiling Water Extensive Or Intensive an extensive property is a property that depends on the amount of matter in a sample. there are two conventions regarding the standard boiling point of water: Tolman coined the terms “intensive” and “extensive” in 1917. Mass and volume are examples of extensive properties. temperature is an intensive variable as, for example, is the density of liquids.. The Temperature Of Boiling Water Extensive Or Intensive.
From www.vernier.com
Boiling Temperature of Water Vernier The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperature is an intensive variable as, for example, is the density of liquids. Here is an explanation of what. Mass and volume are examples of extensive properties. physical chemist and physicist richard c. intensive properties, in contrast, do not depend on the amount of the. The Temperature Of Boiling Water Extensive Or Intensive.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit? HowChimp The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: Mass and volume are examples of extensive properties. Here is an explanation of what. physical chemist and physicist richard c. A quick test to decide whether a. The normal boiling point is commonly given as 100 °c. temperature is an intensive variable as, for example, is. The Temperature Of Boiling Water Extensive Or Intensive.
From www.allrecipes.com
How to Boil Water Faster Allrecipes The Temperature Of Boiling Water Extensive Or Intensive temperature is an intensive variable as, for example, is the density of liquids. Mass and volume are examples of extensive properties. an extensive property is a property that depends on the amount of matter in a sample. physical chemist and physicist richard c. The normal boiling point is commonly given as 100 °c. Here is an explanation. The Temperature Of Boiling Water Extensive Or Intensive.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande's Water Books The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. an extensive property is a property that depends on the amount of matter in a sample. physical chemist and physicist richard c. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. Here is an explanation of what. . The Temperature Of Boiling Water Extensive Or Intensive.
From www.thoughtco.com
The Difference Between Intensive and Extensive Properties The Temperature Of Boiling Water Extensive Or Intensive temperature is an intensive variable as, for example, is the density of liquids. Here is an explanation of what. Tolman coined the terms “intensive” and “extensive” in 1917. physical chemist and physicist richard c. Mass and volume are examples of extensive properties. intensive properties, in contrast, do not depend on the amount of the substance; there. The Temperature Of Boiling Water Extensive Or Intensive.
From exorduurt.blob.core.windows.net
What Is The Temperature Of Boiling Point Of Two Liquid Samples at Erica Walker blog The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. temperature is an intensive variable as, for example, is the density of liquids. Mass and volume are examples of extensive properties. The normal boiling point. The Temperature Of Boiling Water Extensive Or Intensive.
From www.animalia-life.club
Boiling Point Of Water Examples The Temperature Of Boiling Water Extensive Or Intensive intensive properties, in contrast, do not depend on the amount of the substance; Mass and volume are examples of extensive properties. an extensive property is a property that depends on the amount of matter in a sample. there are two conventions regarding the standard boiling point of water: A quick test to decide whether a. physical. The Temperature Of Boiling Water Extensive Or Intensive.
From sciencenotes.org
How to Boil Water at Room Temperature The Temperature Of Boiling Water Extensive Or Intensive intensive properties, in contrast, do not depend on the amount of the substance; temperature is an intensive variable as, for example, is the density of liquids. Here is an explanation of what. Mass and volume are examples of extensive properties. Tolman coined the terms “intensive” and “extensive” in 1917. The normal boiling point is commonly given as 100. The Temperature Of Boiling Water Extensive Or Intensive.
From exokgfcfa.blob.core.windows.net
What Is The Boiling Temperature Of Water In Denver at Belinda Diaz blog The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. The normal boiling point is commonly given as 100 °c. temperature is an intensive variable as, for example, is the density of liquids. an extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. temperatures, density, colour,. The Temperature Of Boiling Water Extensive Or Intensive.
From www.gkseries.com
Temperature of boiling water is measured by a ____ thermometer The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. Mass and volume are examples of extensive properties. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. intensive properties, in contrast, do not depend on the amount of the substance; Tolman coined the terms “intensive” and “extensive” in 1917.. The Temperature Of Boiling Water Extensive Or Intensive.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. physical chemist and physicist richard c. temperature is an intensive variable as, for example, is the density of liquids. Tolman coined the terms “intensive” and “extensive” in 1917. The normal boiling point is commonly given as 100 °c. Mass and volume are examples of extensive properties. intensive properties, in contrast,. The Temperature Of Boiling Water Extensive Or Intensive.
From www.numerade.com
SOLVEDBoiling Water At what temperature would 250 mL of water boil? 1000 mL ? Is the boiling The Temperature Of Boiling Water Extensive Or Intensive temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. A quick test to decide whether a. Mass and volume are examples of extensive properties. The normal boiling point is commonly given as 100 °c. Here is an explanation of what. there are two conventions regarding the. The Temperature Of Boiling Water Extensive Or Intensive.
From www.pinterest.com
Boiling Point Easy Science Easy science, Boiling point, Learn biology The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. Mass and volume are examples of extensive properties. intensive properties, in contrast, do not depend on the amount of the substance; Here is an explanation. The Temperature Of Boiling Water Extensive Or Intensive.
From sciencenotes.org
How to Boil Water at Room Temperature The Temperature Of Boiling Water Extensive Or Intensive an extensive property is a property that depends on the amount of matter in a sample. physical chemist and physicist richard c. A quick test to decide whether a. Mass and volume are examples of extensive properties. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change. The Temperature Of Boiling Water Extensive Or Intensive.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks The Temperature Of Boiling Water Extensive Or Intensive Here is an explanation of what. an extensive property is a property that depends on the amount of matter in a sample. Tolman coined the terms “intensive” and “extensive” in 1917. A quick test to decide whether a. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change. The Temperature Of Boiling Water Extensive Or Intensive.
From exouuujdu.blob.core.windows.net
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita Gafford blog The Temperature Of Boiling Water Extensive Or Intensive temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. physical chemist and physicist richard c. an extensive property is a property that depends on the amount of matter in a sample. there are two conventions regarding the standard boiling point of water: Here is. The Temperature Of Boiling Water Extensive Or Intensive.
From sciencenotes.org
Difference Between Intensive and Extensive Properties of Matter The Temperature Of Boiling Water Extensive Or Intensive physical chemist and physicist richard c. Here is an explanation of what. Mass and volume are examples of extensive properties. Tolman coined the terms “intensive” and “extensive” in 1917. an extensive property is a property that depends on the amount of matter in a sample. The normal boiling point is commonly given as 100 °c. there are. The Temperature Of Boiling Water Extensive Or Intensive.
From studylib.net
Lab Boiling Temperature of Water The Temperature Of Boiling Water Extensive Or Intensive temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will not change with a change in. physical chemist and physicist richard c. Tolman coined the terms “intensive” and “extensive” in 1917. intensive properties, in contrast, do not depend on the amount of the substance; there are two conventions regarding the standard. The Temperature Of Boiling Water Extensive Or Intensive.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Compound Interest The Temperature Of Boiling Water Extensive Or Intensive A quick test to decide whether a. temperature is an intensive variable as, for example, is the density of liquids. Here is an explanation of what. The normal boiling point is commonly given as 100 °c. intensive properties, in contrast, do not depend on the amount of the substance; temperatures, density, colour, melting and boiling point, etc.,. The Temperature Of Boiling Water Extensive Or Intensive.
From waterfilterguru.com
At What Temperature Does Water Boil? The Temperature Of Boiling Water Extensive Or Intensive there are two conventions regarding the standard boiling point of water: Here is an explanation of what. Tolman coined the terms “intensive” and “extensive” in 1917. an extensive property is a property that depends on the amount of matter in a sample. temperatures, density, colour, melting and boiling point, etc., all are intensive properties as they will. The Temperature Of Boiling Water Extensive Or Intensive.