What Pressure Does Water Boil In A Vacuum . 95 rows water boiling point at vacuum pressure. Where can i find the relationship of the boiling and freezing. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. What is the boiling point of water at low pressure ? A vacuum chamber significantly reduces the pressure surrounding liquids. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. water actually boils at a lower temperature if the pressure around it is lowered. This is why if you go to a high altitude location (like. does water freeze at a higher temperature in a vacuum? how vacuum affects pressure. a beaker of water inside of a vacuum chamber boils at room temperature. When water is heated up under vacuum, its boiling point is lower.
from apollo.lsc.vsc.edu
the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. how vacuum affects pressure. What is the boiling point of water at low pressure ? When water is heated up under vacuum, its boiling point is lower. 95 rows water boiling point at vacuum pressure. This is why if you go to a high altitude location (like. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. does water freeze at a higher temperature in a vacuum? water actually boils at a lower temperature if the pressure around it is lowered. a beaker of water inside of a vacuum chamber boils at room temperature.
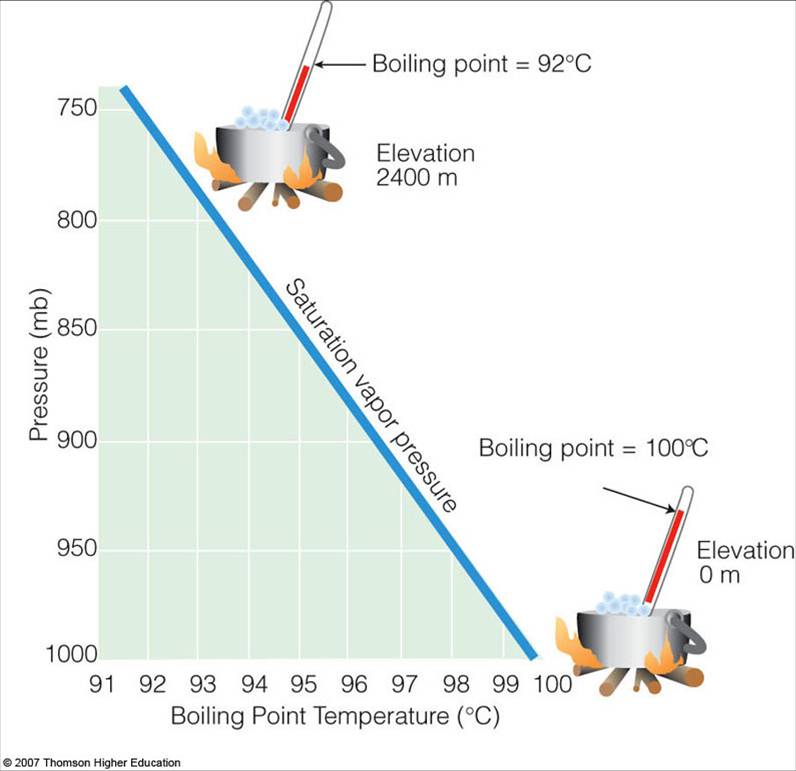
Saturation Vapor Pressure and the Boiling Point
What Pressure Does Water Boil In A Vacuum does water freeze at a higher temperature in a vacuum? This is why if you go to a high altitude location (like. What is the boiling point of water at low pressure ? Where can i find the relationship of the boiling and freezing. A vacuum chamber significantly reduces the pressure surrounding liquids. 95 rows water boiling point at vacuum pressure. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. does water freeze at a higher temperature in a vacuum? a beaker of water inside of a vacuum chamber boils at room temperature. water actually boils at a lower temperature if the pressure around it is lowered. how vacuum affects pressure. When water is heated up under vacuum, its boiling point is lower.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Pressure Does Water Boil In A Vacuum What is the boiling point of water at low pressure ? Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. This is why if you go to a high altitude location (like. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure. What Pressure Does Water Boil In A Vacuum.
From www.researchgate.net
Vacuum pressure (represented as negative values) needed for boiling... Download Scientific Diagram What Pressure Does Water Boil In A Vacuum A vacuum chamber significantly reduces the pressure surrounding liquids. What is the boiling point of water at low pressure ? the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. This is why if you go to a high altitude location (like. Where can i find the. What Pressure Does Water Boil In A Vacuum.
From mavink.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. Where can i find the relationship of the boiling and freezing. When water is heated up under vacuum, its boiling point is. What Pressure Does Water Boil In A Vacuum.
From livingpristine.com
Does Water Boil in a Vacuum Exploring the Effects of Pressure on Boiling Point Living Pristine What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. Where can i find the relationship of the boiling and freezing. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. What is the boiling point. What Pressure Does Water Boil In A Vacuum.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. a beaker of water inside of a vacuum chamber boils at room temperature. When water is heated up under vacuum, its boiling point is lower. water actually boils at a lower temperature if the pressure around it is lowered. the boiling point of water is the temperature at which the vapor pressure. What Pressure Does Water Boil In A Vacuum.
From mungfali.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. 95 rows water boiling point at vacuum pressure. does water freeze at a higher temperature in a vacuum? What is the boiling point of water at low pressure ? water actually boils at a. What Pressure Does Water Boil In A Vacuum.
From livingpristine.com
Does Water Boil in a Vacuum Exploring the Effects of Pressure on Boiling Point Living Pristine What Pressure Does Water Boil In A Vacuum This is why if you go to a high altitude location (like. water actually boils at a lower temperature if the pressure around it is lowered. a beaker of water inside of a vacuum chamber boils at room temperature. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. Where can i. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Pressure Does Water Boil In A Vacuum This is why if you go to a high altitude location (like. A vacuum chamber significantly reduces the pressure surrounding liquids. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. a beaker of water inside of a vacuum chamber boils at room temperature. does water freeze at a higher temperature in. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
Boiling cold water In a Vacuum Chamber YouTube What Pressure Does Water Boil In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. When water is heated up under vacuum, its boiling point is lower. A vacuum chamber significantly reduces the pressure surrounding liquids. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the.. What Pressure Does Water Boil In A Vacuum.
From mavink.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum This is why if you go to a high altitude location (like. how vacuum affects pressure. Where can i find the relationship of the boiling and freezing. What is the boiling point of water at low pressure ? a beaker of water inside of a vacuum chamber boils at room temperature. Online calculator, figures and tables giving the. What Pressure Does Water Boil In A Vacuum.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Experiments What Pressure Does Water Boil In A Vacuum the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. does water freeze at a higher temperature in a vacuum? a beaker of water inside of a vacuum chamber. What Pressure Does Water Boil In A Vacuum.
From tedkinsman.photoshelter.com
Boiling Ice Water in a Vacuum What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. does water freeze at a higher temperature in a vacuum? the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. When water is heated up. What Pressure Does Water Boil In A Vacuum.
From mavink.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum does water freeze at a higher temperature in a vacuum? When water is heated up under vacuum, its boiling point is lower. 95 rows water boiling point at vacuum pressure. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. Where can i find the. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
What happens to water inside a vacuum chamber? YouTube What Pressure Does Water Boil In A Vacuum a beaker of water inside of a vacuum chamber boils at room temperature. A vacuum chamber significantly reduces the pressure surrounding liquids. This is why if you go to a high altitude location (like. how vacuum affects pressure. does water freeze at a higher temperature in a vacuum? Where can i find the relationship of the boiling. What Pressure Does Water Boil In A Vacuum.
From mungfali.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. What is the boiling point of water at low pressure ? does water freeze at a higher temperature in a vacuum? Where can i find the relationship of the boiling and freezing. When water is heated up under vacuum, its boiling point is lower.. What Pressure Does Water Boil In A Vacuum.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. Where can i find the relationship of the boiling and freezing. A vacuum chamber significantly reduces the pressure surrounding liquids. 95 rows water boiling point at vacuum pressure. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. What is the boiling point of water at low pressure. What Pressure Does Water Boil In A Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature What Pressure Does Water Boil In A Vacuum This is why if you go to a high altitude location (like. When water is heated up under vacuum, its boiling point is lower. a beaker of water inside of a vacuum chamber boils at room temperature. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding. What Pressure Does Water Boil In A Vacuum.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz What Pressure Does Water Boil In A Vacuum the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. A vacuum chamber significantly reduces the pressure surrounding liquids. When water is heated up under vacuum, its boiling point is lower.. What Pressure Does Water Boil In A Vacuum.
From livingpristine.com
Why Does Water Boil in a Vacuum? Exploring The Fascinating Phenomenon Living Pristine What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. When water is heated up under vacuum, its boiling point is lower. 95 rows water boiling point at vacuum pressure. What is the boiling point of water at low pressure ? A vacuum chamber significantly reduces the pressure surrounding liquids. This is why if you go to a high altitude location (like. a. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation YouTube What Pressure Does Water Boil In A Vacuum Where can i find the relationship of the boiling and freezing. This is why if you go to a high altitude location (like. does water freeze at a higher temperature in a vacuum? how vacuum affects pressure. What is the boiling point of water at low pressure ? Online calculator, figures and tables giving the boiling temperatures of. What Pressure Does Water Boil In A Vacuum.
From mavink.com
Water Vapour Pressure Chart What Pressure Does Water Boil In A Vacuum the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. does water freeze at a higher temperature in a vacuum? 95 rows water boiling point at vacuum pressure. A vacuum chamber significantly reduces the pressure surrounding liquids. When water is heated up under vacuum, its. What Pressure Does Water Boil In A Vacuum.
From www.slideserve.com
PPT Chapter 13 States of Matter PowerPoint Presentation, free download ID5118609 What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. This is why if you go to a high altitude location (like. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. When water is heated up under vacuum, its boiling point is lower. a beaker of water. What Pressure Does Water Boil In A Vacuum.
From scindustrialsales.com
Boiling Point of Water Under Vacuum Pumps and Filtration What Pressure Does Water Boil In A Vacuum 95 rows water boiling point at vacuum pressure. What is the boiling point of water at low pressure ? a beaker of water inside of a vacuum chamber boils at room temperature. Where can i find the relationship of the boiling and freezing. the boiling point of water is the temperature at which the vapor pressure of. What Pressure Does Water Boil In A Vacuum.
From dxofqxdff.blob.core.windows.net
Vacuum Distillation Column Pressure at Joseph Croley blog What Pressure Does Water Boil In A Vacuum A vacuum chamber significantly reduces the pressure surrounding liquids. This is why if you go to a high altitude location (like. how vacuum affects pressure. does water freeze at a higher temperature in a vacuum? Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. What is the boiling point of water. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
boiling water in vacuum chamber YouTube What Pressure Does Water Boil In A Vacuum 95 rows water boiling point at vacuum pressure. how vacuum affects pressure. When water is heated up under vacuum, its boiling point is lower. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. the boiling point of water is the temperature at which the vapor pressure of the liquid water. What Pressure Does Water Boil In A Vacuum.
From mavink.com
Water Boiling Point Vs Pressure Chart What Pressure Does Water Boil In A Vacuum A vacuum chamber significantly reduces the pressure surrounding liquids. does water freeze at a higher temperature in a vacuum? When water is heated up under vacuum, its boiling point is lower. Where can i find the relationship of the boiling and freezing. 95 rows water boiling point at vacuum pressure. how vacuum affects pressure. Online calculator, figures. What Pressure Does Water Boil In A Vacuum.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition, Conditions, Characteristics What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. A vacuum chamber significantly reduces the pressure surrounding liquids. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. a beaker of water inside of a vacuum chamber boils at room temperature. Where can i find the relationship. What Pressure Does Water Boil In A Vacuum.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Pressure Does Water Boil In A Vacuum Where can i find the relationship of the boiling and freezing. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. What is the boiling point of water at low pressure ? water actually boils at a lower temperature if the pressure around it is lowered. does water freeze at a higher. What Pressure Does Water Boil In A Vacuum.
From gfecc.org
Gallery of liquid ring vacuum pump working principle and pumping system vacuum temperature What Pressure Does Water Boil In A Vacuum Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. water actually boils at a lower temperature if the pressure around it is lowered. A vacuum chamber significantly reduces the pressure surrounding liquids. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
Does Water Really Boil in a Vacuum Chamber? And Why? YouTube What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. how vacuum affects pressure. 95 rows water boiling point at vacuum pressure. Where can i find the relationship of the boiling and freezing. This is why if you go to a high altitude location (like. A vacuum chamber significantly reduces the pressure surrounding. What Pressure Does Water Boil In A Vacuum.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience What Pressure Does Water Boil In A Vacuum water actually boils at a lower temperature if the pressure around it is lowered. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. a beaker of water inside of a vacuum chamber boils at room temperature. When water is heated up under vacuum, its boiling point is lower. A vacuum chamber. What Pressure Does Water Boil In A Vacuum.
From mungfali.com
Water Boiling Point Pressure Chart What Pressure Does Water Boil In A Vacuum 95 rows water boiling point at vacuum pressure. how vacuum affects pressure. A vacuum chamber significantly reduces the pressure surrounding liquids. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. When water is heated up under vacuum, its boiling point is lower. Where can i find the relationship of the boiling. What Pressure Does Water Boil In A Vacuum.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Pressure Does Water Boil In A Vacuum how vacuum affects pressure. 95 rows water boiling point at vacuum pressure. This is why if you go to a high altitude location (like. water actually boils at a lower temperature if the pressure around it is lowered. What is the boiling point of water at low pressure ? When water is heated up under vacuum, its. What Pressure Does Water Boil In A Vacuum.
From www.youtube.com
Why does water boil in a vacuum? YouTube What Pressure Does Water Boil In A Vacuum A vacuum chamber significantly reduces the pressure surrounding liquids. how vacuum affects pressure. water actually boils at a lower temperature if the pressure around it is lowered. the boiling point of water is the temperature at which the vapor pressure of the liquid water equals the pressure surrounding the. a beaker of water inside of a. What Pressure Does Water Boil In A Vacuum.
From sciencenotes.org
How to Boil Water at Room Temperature What Pressure Does Water Boil In A Vacuum What is the boiling point of water at low pressure ? how vacuum affects pressure. A vacuum chamber significantly reduces the pressure surrounding liquids. a beaker of water inside of a vacuum chamber boils at room temperature. Online calculator, figures and tables giving the boiling temperatures of water in varying vacuum, si and. This is why if you. What Pressure Does Water Boil In A Vacuum.