Magnesium Hydrogen Carbonate Solubility . it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate is slightly soluble. There is little data for. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium.
from www.chegg.com
There is little data for. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium carbonate is slightly soluble. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2].
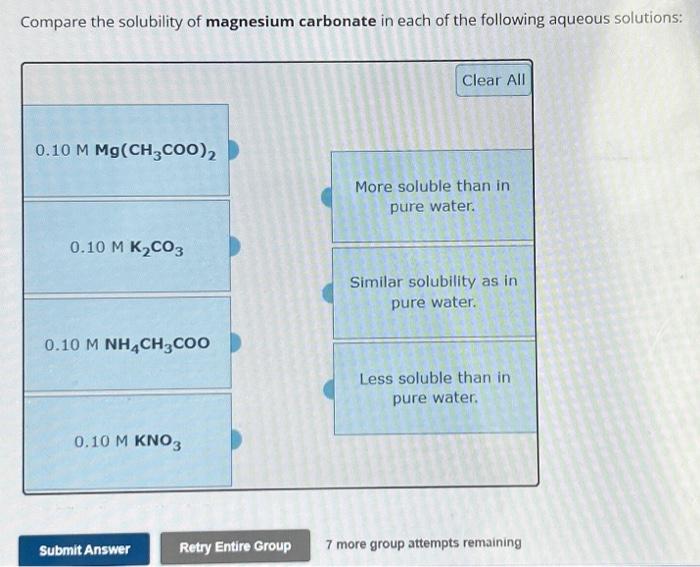
Solved Compare the solubility of magnesium carbonate in each
Magnesium Hydrogen Carbonate Solubility magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. There is little data for. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium carbonate is slightly soluble. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1].
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Magnesium Hydrogen Carbonate Solubility magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium carbonate is slightly soluble. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c. Magnesium Hydrogen Carbonate Solubility.
From oneclass.com
OneClass of the magnesium carbonate in Compare the solubility Magnesium Hydrogen Carbonate Solubility Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. There is little data for. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium carbonate is slightly soluble. magnesium carbonate, for example,. Magnesium Hydrogen Carbonate Solubility.
From www.researchgate.net
Hydrogen solubility in pure magnesium [21]. Download Scientific Diagram Magnesium Hydrogen Carbonate Solubility Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. There is little data for. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. . Magnesium Hydrogen Carbonate Solubility.
From oneclass.com
OneClass of the magnesium carbonate in Compare the solubility Magnesium Hydrogen Carbonate Solubility Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. There is little data for. magnesium bicarbonate,. Magnesium Hydrogen Carbonate Solubility.
From www.fishersci.com
Magnesium carbonate hydroxide pentahydrate, light, 98, Thermo Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100. Magnesium Hydrogen Carbonate Solubility.
From pubs.acs.org
Solubility of Amorphous Magnesium Carbonate at Low Temperatures Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. There is little data for. magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Many hydrogen carbonates, such. Magnesium Hydrogen Carbonate Solubility.
From hydrogenpotanezu.blogspot.com
Hydrogen Magnesium Hydrogen Carbonate Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2. Magnesium Hydrogen Carbonate Solubility.
From www.numerade.com
SOLVED The concentrations of magnesium and carbonate ions in a Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c. Magnesium Hydrogen Carbonate Solubility.
From www.researchgate.net
Normalized hydrogen solubility from the literature for AlMgAlSi Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. There is little data for. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented. Magnesium Hydrogen Carbonate Solubility.
From chem.libretexts.org
13.3 Factors Affecting Solubility Chemistry LibreTexts Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. There is little data for. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium bicarbonate or. Magnesium Hydrogen Carbonate Solubility.
From www.researchgate.net
2) gives the solubility of magnesium in water at 25°C as a function of Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium bicarbonate, also known. Magnesium Hydrogen Carbonate Solubility.
From sciencelab-limited.myshopify.com
Magnesium Hydrogen Carbonate Sciencelab limited Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. There is little data for. Since magnesium bicarbonate is unstable in a solid state, it exists in. Magnesium Hydrogen Carbonate Solubility.
From hydrogenpotanezu.blogspot.com
Hydrogen Magnesium Hydrogen Carbonate Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are. Magnesium Hydrogen Carbonate Solubility.
From www.slideserve.com
PPT Dissolution and Precipitation PowerPoint Presentation, free Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. There is little data for. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate, for example, has a. Magnesium Hydrogen Carbonate Solubility.
From pubs.rsc.org
Solubility investigations in the amorphous calcium magnesium carbonate Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. it is formed by the action. Magnesium Hydrogen Carbonate Solubility.
From pubs.acs.org
Control of Water Chemistry in Alkaline Lakes Solubility of Magnesium Hydrogen Carbonate Solubility There is little data for. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. . Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. There is little data for. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Since magnesium bicarbonate is unstable in a solid state, it exists in a. Magnesium Hydrogen Carbonate Solubility.
From www.youtube.com
How to Write the Formula for Magnesium bicarbonate (Magnesium hydrogen Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. There is little data for. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. Many hydrogen. Magnesium Hydrogen Carbonate Solubility.
From pressbooks.online.ucf.edu
12.3 Types of Solutions and Solubility Chemistry Fundamentals Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. There is little data for. magnesium carbonate, for example, has a solubility of. Magnesium Hydrogen Carbonate Solubility.
From h-o-m-e.org
Solubility of Carbonates Defined Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. There is little data for. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Calculate the solubility of magnesium carbonate at 25 Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. magnesium carbonate is slightly soluble. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate is slightly soluble. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
of the magnesium carbonate in Compare the solubility Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility There is little data for. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. magnesium carbonate is slightly soluble. magnesium. Magnesium Hydrogen Carbonate Solubility.
From testbook.com
Magnesium Carbonate Learn Definition, Structure, Formula & Uses Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium carbonate is slightly soluble. There is little data for. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate,. Magnesium Hydrogen Carbonate Solubility.
From www.numerade.com
SOLVEDCalculate The K sp of magnesium carbonate (Mg CO3) is 2.6 ×10^9 Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium carbonate is slightly soluble. . Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved What is the molar solubility of magnesium carbonate ( Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at. Magnesium Hydrogen Carbonate Solubility.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Magnesium Hydrogen Carbonate Solubility it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room temperature. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt. Magnesium Hydrogen Carbonate Solubility.
From www.numerade.com
SOLVED Write a balanced chemical equation for the following Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. it is formed by the action. Magnesium Hydrogen Carbonate Solubility.
From www.youtube.com
The molar solubility of magnesium carbonate is 1.87x10^4 mol/L Magnesium Hydrogen Carbonate Solubility magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. magnesium carbonate is slightly soluble. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. There is little data for. magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt. Magnesium Hydrogen Carbonate Solubility.
From techiescience.com
Comprehensive Guide to Magnesium Carbonate Solubility A Detailed Magnesium Hydrogen Carbonate Solubility magnesium carbonate is slightly soluble. Many hydrogen carbonates, such as ca(hco3)2 and mg(hco3)2, are soluble. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate, for example, has a solubility of about 0.02 g per 100 g of water at room. Magnesium Hydrogen Carbonate Solubility.
From www.chegg.com
Solved 18. What is the solubility of magnesium carbonate, Magnesium Hydrogen Carbonate Solubility There is little data for. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2]. it is formed by the action of carbon dioxide on a suspension of magnesium carbonate in water:mgco 3 (s)+co 2 (g)+h 2 o(l) →. magnesium carbonate is slightly soluble. magnesium carbonate, for example, has a. Magnesium Hydrogen Carbonate Solubility.