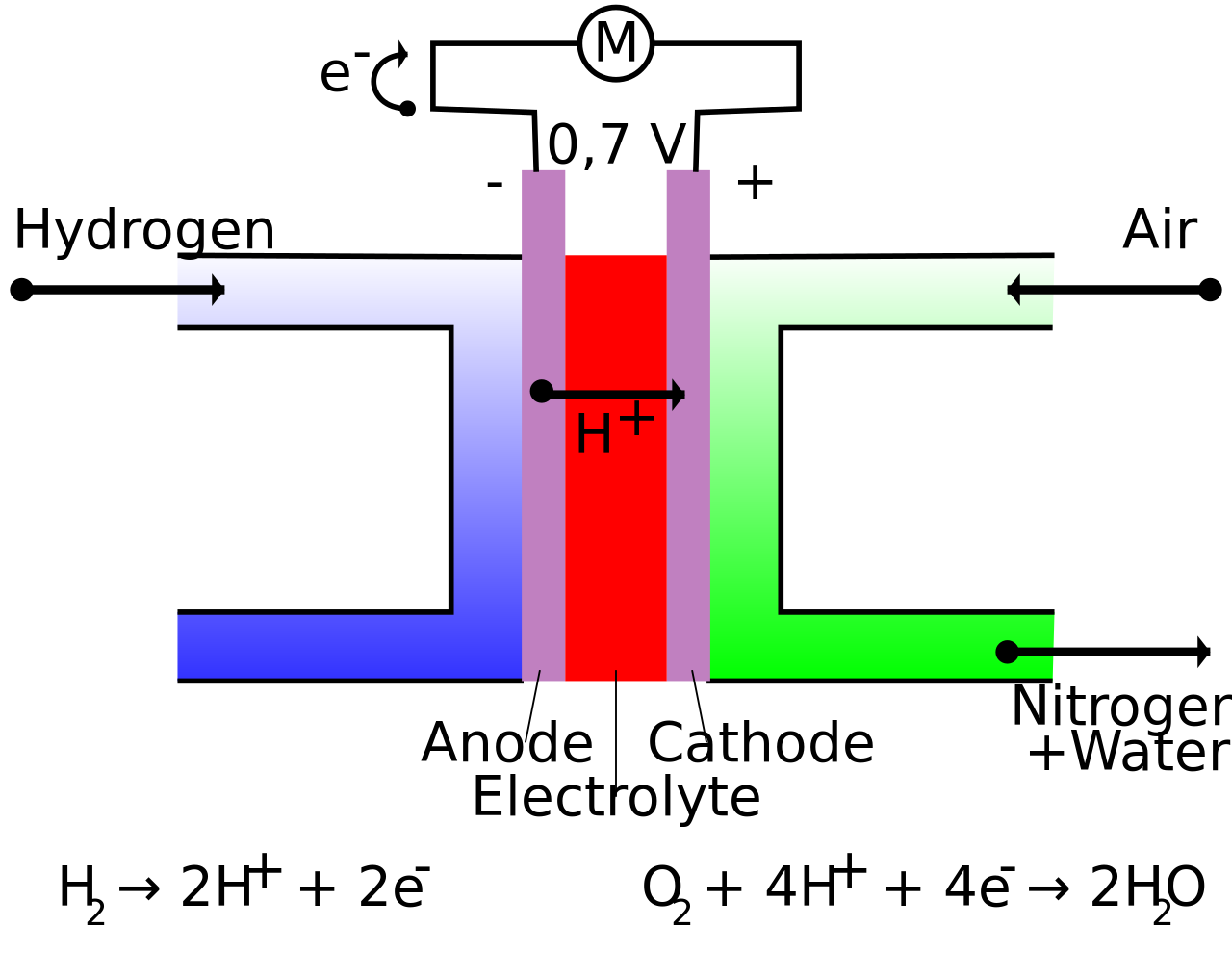
Fuel Chemistry Equation . Hydrogen ions combine with 02. — what is fuel? revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. There will be some quizzes and homework. One of the most important applications of chemistry is the study of fuels. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Into the cell and flows over the anode catalyst. The big picture on fuels and combustion. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
from large.stanford.edu
There will be some quizzes and homework. — what is fuel? Hydrogen ions combine with 02. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. Into the cell and flows over the anode catalyst. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. One of the most important applications of chemistry is the study of fuels. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen.
Hydrogen Fuel Cells
Fuel Chemistry Equation revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. The big picture on fuels and combustion. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. Hydrogen ions combine with 02. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. There will be some quizzes and homework. One of the most important applications of chemistry is the study of fuels. — what is fuel?
From butchixanh.edu.vn
Combined Gas Law Definition, Formula, Examples Bút Chì Xanh Fuel Chemistry Equation revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture. Fuel Chemistry Equation.
From chem.libretexts.org
3.8 Gasoline A Deeper Look Chemistry LibreTexts Fuel Chemistry Equation Into the cell and flows over the anode catalyst. Hydrogen ions combine with 02. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — what is fuel? fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and. Fuel Chemistry Equation.
From www.shutterstock.com
Gas Reaction Equation Gas Evolution Reaction Stock Vector (Royalty Free Fuel Chemistry Equation — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. One of the most important applications of chemistry is the study. Fuel Chemistry Equation.
From www.youtube.com
AP Chemistry Gas Density YouTube Fuel Chemistry Equation There will be some quizzes and homework. — what is fuel? Into the cell and flows over the anode catalyst. The big picture on fuels and combustion. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. One of the most important applications of chemistry is. Fuel Chemistry Equation.
From www.researchgate.net
(PDF) Combustion of fuels Fuel Chemistry Equation revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The big picture on fuels and combustion. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. Hydrogen ions combine with 02. — what is fuel? One. Fuel Chemistry Equation.
From dxokdgtal.blob.core.windows.net
How Do You Calculate Moles Of Gas at Bobby Andersen blog Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. One of the most important applications of chemistry is the study of fuels. Hydrogen ions combine with 02. There will be some quizzes and homework. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by. Fuel Chemistry Equation.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Fuel Chemistry Equation — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. One of the most important applications of chemistry is the study of fuels. fuel cells close fuel. Fuel Chemistry Equation.
From mmerevise.co.uk
The Ideal Gas Equation MME Fuel Chemistry Equation One of the most important applications of chemistry is the study of fuels. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen ions combine with 02. There will be some quizzes and homework. fuel cells close fuel cell device that produces a voltage continuously. Fuel Chemistry Equation.
From www.dreamstime.com
Fuel Molecules set stock vector. Illustration of decane 76462009 Fuel Chemistry Equation The big picture on fuels and combustion. — what is fuel? when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell. Fuel Chemistry Equation.
From revisechemistry.uk
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — what is fuel? — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. One. Fuel Chemistry Equation.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Fuel Chemistry Equation fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. One of the most important applications of chemistry is the study of fuels. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. Into the cell and flows. Fuel Chemistry Equation.
From solvedlib.com
Rocket Fuel The exothermic reaction between liquid h… SolvedLib Fuel Chemistry Equation There will be some quizzes and homework. — what is fuel? revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The big picture on fuels and combustion. — a fuel cell like this will continue to operate and produce electrical energy as long as. Fuel Chemistry Equation.
From en.ppt-online.org
The ideal gas equation online presentation Fuel Chemistry Equation Into the cell and flows over the anode catalyst. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. There will be some quizzes and homework. One of. Fuel Chemistry Equation.
From www.alamy.com
Ideal gas law formula in chemistry. Chemistry resources for teachers Fuel Chemistry Equation fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The big picture on fuels and combustion. — in the most common approach to. Fuel Chemistry Equation.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Fuel Chemistry Equation — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen ions combine with 02. — what is fuel? The big picture on fuels and combustion. — in the most common approach to coal gasification, coal reacts with steam to produce a. Fuel Chemistry Equation.
From www.tessshebaylo.com
Chemical Equation Synthesis Tessshebaylo Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. One of the most important applications of chemistry is the study of fuels. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. revision. Fuel Chemistry Equation.
From sciencenotes.org
Combustion Reaction Definition and Examples Fuel Chemistry Equation Hydrogen ions combine with 02. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Into the cell and flows over the anode catalyst. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known.. Fuel Chemistry Equation.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Chemistry Equation There will be some quizzes and homework. — what is fuel? fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. when fuels burn they release. Fuel Chemistry Equation.
From www.youtube.com
Ideal gas equation units R MDCAT YouTube Fuel Chemistry Equation The big picture on fuels and combustion. — what is fuel? — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Hydrogen ions combine with 02. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions. Fuel Chemistry Equation.
From www.youtube.com
Gas volumes (AQA GCSE Chemistry) YouTube Fuel Chemistry Equation — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. Into the cell and flows over the anode catalyst. revision notes on 5.4.6 fuel cells for the. Fuel Chemistry Equation.
From www.linstitute.net
IB DP Chemistry SL复习笔记1.2.3 Avogadro's Law & Molar Gas Volume翰林国际教育 Fuel Chemistry Equation fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. — what is fuel? revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen ions combine with 02. — in the most common approach to. Fuel Chemistry Equation.
From www.slideshare.net
Organic chemistry fossil fuels Fuel Chemistry Equation There will be some quizzes and homework. The big picture on fuels and combustion. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Into the cell and flows over the anode catalyst. revision notes on 5.4.6 fuel cells for the aqa a. Fuel Chemistry Equation.
From www.tes.com
Molar Volume of Gases GCSE Lesson (SC14e) TRIPLE Teaching Resources Fuel Chemistry Equation There will be some quizzes and homework. One of the most important applications of chemistry is the study of fuels. — what is fuel? Hydrogen ions combine with 02. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. The big picture on fuels and combustion.. Fuel Chemistry Equation.
From exowshemi.blob.core.windows.net
Fuels Chemical Composition at Megan Ureno blog Fuel Chemistry Equation — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. Into the cell and flows over the anode catalyst. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. revision notes on 5.4.6 fuel cells for the. Fuel Chemistry Equation.
From mungfali.com
Gas Laws Formula Sheet Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. — in the most common approach to coal gasification, coal reacts with steam to. Fuel Chemistry Equation.
From www.tes.com
Physical Chemistry 12 The Gaseous State, Ideal Gas Law and General Fuel Chemistry Equation There will be some quizzes and homework. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. revision notes on 5.4.6 fuel cells for. Fuel Chemistry Equation.
From www.youtube.com
Molar Mass of a Gas at STP Equations & Formulas, Chemistry Practice Fuel Chemistry Equation One of the most important applications of chemistry is the study of fuels. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. Hydrogen ions combine with 02. Into the cell and flows over the anode catalyst. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus,. Fuel Chemistry Equation.
From www.wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. The big picture on fuels and combustion. — in the most common approach to coal gasification, coal reacts with steam to. Fuel Chemistry Equation.
From bdteletalk.com
Equation For The Combustion Of Methane Fuel Chemistry Equation when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The big picture on fuels and combustion. Into the cell and flows over the anode. Fuel Chemistry Equation.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Chemistry Equation Into the cell and flows over the anode catalyst. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. The big picture on fuels and combustion. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hydrogen ions. Fuel Chemistry Equation.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Fuel Chemistry Equation There will be some quizzes and homework. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. One of the most important applications of chemistry is the study. Fuel Chemistry Equation.
From www.youtube.com
GCSE Science Chemistry (91 Triple) Fuel Cells YouTube Fuel Chemistry Equation revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. One of the most important applications of chemistry is the study of fuels. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. . Fuel Chemistry Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Fuel Chemistry Equation revision notes on 5.4.6 fuel cells for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Into the cell and flows over the anode catalyst. One of the most important applications of chemistry is the study of fuels. — what is fuel? The big picture on fuels and combustion. Hydrogen ions combine. Fuel Chemistry Equation.
From chem.libretexts.org
3.9 Gasoline A Deeper Look Chemistry LibreTexts Fuel Chemistry Equation The big picture on fuels and combustion. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. Into the cell and flows over the anode catalyst. One of. Fuel Chemistry Equation.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Fuel Chemistry Equation Hydrogen ions combine with 02. when fuels burn they release heat energy and light energy to the surroundings in exothermic reactions known as. — in the most common approach to coal gasification, coal reacts with steam to produce a mixture of co and h2 known. — a fuel cell like this will continue to operate and produce. Fuel Chemistry Equation.