How Can I Find The Heat Capacity Of A Calorimeter . — the temperature change produced by the known reaction is used to determine the heat capacity of the. Figure out how to find the heat. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. The temperature change measured by the calorimeter is used to derive the. It can analyze the heat exchange between up to 3 objects. to do so, the heat is exchanged with a calibrated object (calorimeter). — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. 370k views 5 years ago 1 product. — the calorimetry calculator can help you solve complex calorimetry problems.
from www.tec-science.com
— the temperature change produced by the known reaction is used to determine the heat capacity of the. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. to do so, the heat is exchanged with a calibrated object (calorimeter). It can analyze the heat exchange between up to 3 objects. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. Figure out how to find the heat. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. The temperature change measured by the calorimeter is used to derive the. 370k views 5 years ago 1 product. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\).
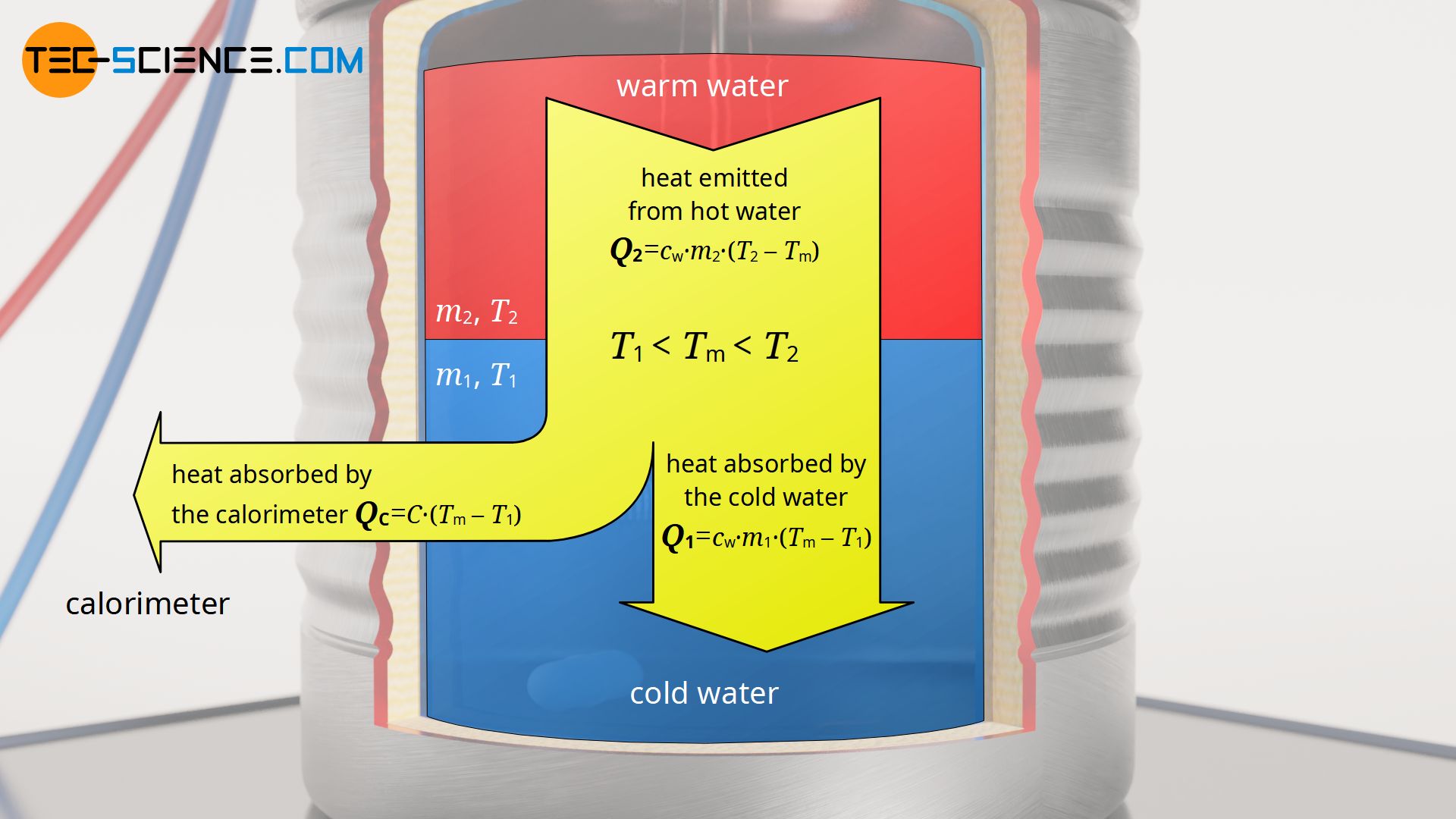
Calorimeter to determine the specific heat capacities of liquids tec
How Can I Find The Heat Capacity Of A Calorimeter — the calorimetry calculator can help you solve complex calorimetry problems. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. 370k views 5 years ago 1 product. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). It can analyze the heat exchange between up to 3 objects. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. to do so, the heat is exchanged with a calibrated object (calorimeter). The temperature change measured by the calorimeter is used to derive the. — the temperature change produced by the known reaction is used to determine the heat capacity of the. — the calorimetry calculator can help you solve complex calorimetry problems. Figure out how to find the heat.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo How Can I Find The Heat Capacity Of A Calorimeter to do so, the heat is exchanged with a calibrated object (calorimeter). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). — the temperature. How Can I Find The Heat Capacity Of A Calorimeter.
From haipernews.com
How To Calculate Joules Of Heat Released Haiper How Can I Find The Heat Capacity Of A Calorimeter — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 370k views 5 years ago 1 product. It can analyze the heat exchange between up to 3 objects. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube How Can I Find The Heat Capacity Of A Calorimeter The temperature change measured by the calorimeter is used to derive the. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount. How Can I Find The Heat Capacity Of A Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Can I Find The Heat Capacity Of A Calorimeter — the temperature change produced by the known reaction is used to determine the heat capacity of the. Figure out how to find the heat. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The temperature change measured by the calorimeter is used to derive the. 370k views 5. How Can I Find The Heat Capacity Of A Calorimeter.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer How Can I Find The Heat Capacity Of A Calorimeter — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. 370k views 5 years ago 1 product. — the calorimetry. How Can I Find The Heat Capacity Of A Calorimeter.
From www.chegg.com
Solved connect Calorimetry TRY. HEAT How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. It can analyze the heat exchange between up to 3 objects. 370k views 5 years ago 1 product. — the temperature change produced by the known reaction is used to determine the heat capacity of the. Figure out how. How Can I Find The Heat Capacity Of A Calorimeter.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , How Can I Find The Heat Capacity Of A Calorimeter — the temperature change produced by the known reaction is used to determine the heat capacity of the. — the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Figure out how to find the heat. to do so, the heat is exchanged with a calibrated. How Can I Find The Heat Capacity Of A Calorimeter.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. 370k views 5 years ago 1 product. to do so, the heat is exchanged with. How Can I Find The Heat Capacity Of A Calorimeter.
From learningschoolsmmnifl.z4.web.core.windows.net
Heat Of Reaction Chemistry How Can I Find The Heat Capacity Of A Calorimeter — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. It can analyze the heat exchange between up to 3 objects. . How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How Can I Find The Heat Capacity Of A Calorimeter — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. Figure out how to find the heat. It can analyze the heat exchange between up to 3 objects. the heat capacity of the calorimeter or of the reaction mixture may. How Can I Find The Heat Capacity Of A Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Can I Find The Heat Capacity Of A Calorimeter 370k views 5 years ago 1 product. Figure out how to find the heat. — the calorimetry calculator can help you solve complex calorimetry problems. — the temperature change produced by the known reaction is used to determine the heat capacity of the. — normally it can be done by heating a piece of nickel or something,. How Can I Find The Heat Capacity Of A Calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download How Can I Find The Heat Capacity Of A Calorimeter The temperature change measured by the calorimeter is used to derive the. — the temperature change produced by the known reaction is used to determine the heat capacity of the. to do so, the heat is exchanged with a calibrated object (calorimeter). — normally it can be done by heating a piece of nickel or something, recording. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube How Can I Find The Heat Capacity Of A Calorimeter — the calorimetry calculator can help you solve complex calorimetry problems. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). to do so, the heat is exchanged with a calibrated object (calorimeter). the heat capacity of the calorimeter or of the reaction mixture may be used to. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube How Can I Find The Heat Capacity Of A Calorimeter 370k views 5 years ago 1 product. to do so, the heat is exchanged with a calibrated object (calorimeter). — the calorimetry calculator can help you solve complex calorimetry problems. Figure out how to find the heat. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. . How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
Calorimetry calculation YouTube How Can I Find The Heat Capacity Of A Calorimeter — the temperature change produced by the known reaction is used to determine the heat capacity of the. Figure out how to find the heat. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. — then use equation 5.6.21 to determine the heat capacity of the calorimeter. How Can I Find The Heat Capacity Of A Calorimeter.
From keystagewiki.com
GCSE Physics Required Practical Determining Specific Heat Capacity How Can I Find The Heat Capacity Of A Calorimeter the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — the temperature change produced by the known reaction is used to determine the heat capacity of the. — the calorimetry calculator can help you solve complex calorimetry problems. — then use equation. How Can I Find The Heat Capacity Of A Calorimeter.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates How Can I Find The Heat Capacity Of A Calorimeter — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. 370k views 5 years ago 1 product. — the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. . How Can I Find The Heat Capacity Of A Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Can I Find The Heat Capacity Of A Calorimeter Figure out how to find the heat. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — the calorimetry calculator can help you solve complex. How Can I Find The Heat Capacity Of A Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How Can I Find The Heat Capacity Of A Calorimeter Figure out how to find the heat. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. to do so, the heat is exchanged with a calibrated object (calorimeter). . How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
Specific heat capacity YouTube How Can I Find The Heat Capacity Of A Calorimeter Figure out how to find the heat. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The temperature change measured by. How Can I Find The Heat Capacity Of A Calorimeter.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. — then use equation 5.6.21 to determine the heat capacity. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How Can I Find The Heat Capacity Of A Calorimeter It can analyze the heat exchange between up to 3 objects. — the calorimetry calculator can help you solve complex calorimetry problems. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. — the temperature change produced by the. How Can I Find The Heat Capacity Of A Calorimeter.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Figure out how to find the heat. 370k views 5 years ago 1 product. — the temperature change produced by. How Can I Find The Heat Capacity Of A Calorimeter.
From www.numerade.com
SOLVED Can somebody explain how to find the heat absorbed by a How Can I Find The Heat Capacity Of A Calorimeter — the temperature change produced by the known reaction is used to determine the heat capacity of the. — the calorimetry calculator can help you solve complex calorimetry problems. Figure out how to find the heat. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. —. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube How Can I Find The Heat Capacity Of A Calorimeter It can analyze the heat exchange between up to 3 objects. the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — the temperature change produced by the known reaction is used to determine the heat capacity of the. — i need to find. How Can I Find The Heat Capacity Of A Calorimeter.
From www.studocu.com
Procedures IN Determining THE HEAT Capacity OF A Calorimeter A How Can I Find The Heat Capacity Of A Calorimeter — the temperature change produced by the known reaction is used to determine the heat capacity of the. — the calorimetry calculator can help you solve complex calorimetry problems. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The temperature change measured by the calorimeter is used to. How Can I Find The Heat Capacity Of A Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter How Can I Find The Heat Capacity Of A Calorimeter The temperature change measured by the calorimeter is used to derive the. 370k views 5 years ago 1 product. Figure out how to find the heat. to do so, the heat is exchanged with a calibrated object (calorimeter). — the calorimetry calculator can help you solve complex calorimetry problems. — then use equation 5.6.21 to determine the. How Can I Find The Heat Capacity Of A Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. — the calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. Figure out how to find the heat. The temperature change measured by the calorimeter is used to. How Can I Find The Heat Capacity Of A Calorimeter.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The How Can I Find The Heat Capacity Of A Calorimeter — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. The temperature change measured by the calorimeter is used to derive the. — the calorimetry calculator can help you solve complex calorimetry problems. the heat capacity of the calorimeter or of the reaction mixture may be used to. How Can I Find The Heat Capacity Of A Calorimeter.
From sites.google.com
Calorimetry Preliminary HSC Chemistry How Can I Find The Heat Capacity Of A Calorimeter the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. — the temperature change produced by. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry How Can I Find The Heat Capacity Of A Calorimeter to do so, the heat is exchanged with a calibrated object (calorimeter). the heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. Figure out how to find the heat. — the temperature change produced by the known reaction is used to determine the heat. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube How Can I Find The Heat Capacity Of A Calorimeter — the calorimetry calculator can help you solve complex calorimetry problems. 370k views 5 years ago 1 product. to do so, the heat is exchanged with a calibrated object (calorimeter). — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. The temperature change measured by the calorimeter is. How Can I Find The Heat Capacity Of A Calorimeter.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals How Can I Find The Heat Capacity Of A Calorimeter It can analyze the heat exchange between up to 3 objects. — the calorimetry calculator can help you solve complex calorimetry problems. — the temperature change produced by the known reaction is used to determine the heat capacity of the. — then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and. How Can I Find The Heat Capacity Of A Calorimeter.
From www.youtube.com
050 Calorimetry YouTube How Can I Find The Heat Capacity Of A Calorimeter — the calorimetry calculator can help you solve complex calorimetry problems. — i need to find the heat lost of an unknown metal dropped into a calorimeter with $70~\mathrm{g}$ $\ce{h2o}$. — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the. How Can I Find The Heat Capacity Of A Calorimeter.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical How Can I Find The Heat Capacity Of A Calorimeter — normally it can be done by heating a piece of nickel or something, recording the temperature of the metal and the water, and then dropping the metal. 370k views 5 years ago 1 product. The temperature change measured by the calorimeter is used to derive the. — the temperature change produced by the known reaction is used. How Can I Find The Heat Capacity Of A Calorimeter.