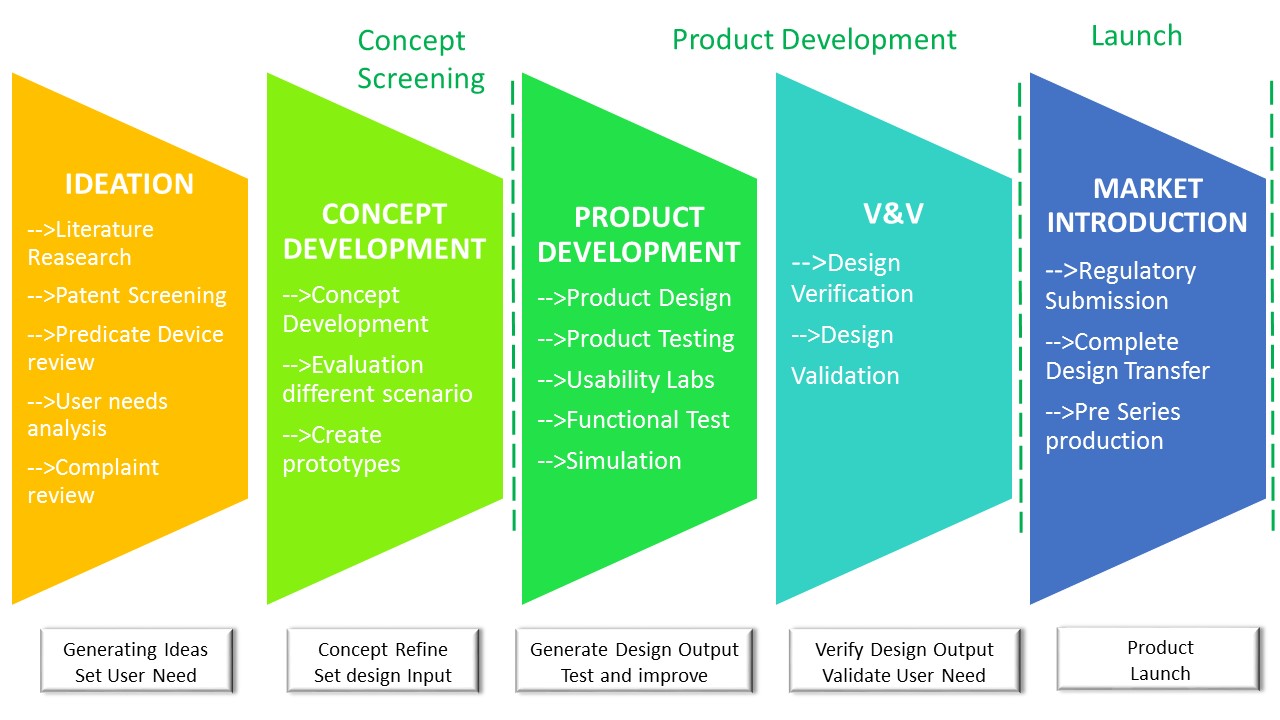
Medical Device Design Input Examples . Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Needs and expectations of business, clinical, and user stakeholders. Let’s look at a basic example: With this guide, i plan to. Well established design inputs can make the rest of. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Design inputs are essential for medical device development. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this.
from tsqasia.com
Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Needs and expectations of business, clinical, and user stakeholders. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Well established design inputs can make the rest of. Design inputs are essential for medical device development. Let’s look at a basic example: With this guide, i plan to. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Design inputs are supposed to be objective criteria for verification that the design outputs are.
Medical Devices EU Design Control Process Support TSQ Middle East & Asia
Medical Device Design Input Examples With this guide, i plan to. Design inputs are essential for medical device development. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Needs and expectations of business, clinical, and user stakeholders. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. With this guide, i plan to. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Let’s look at a basic example: Well established design inputs can make the rest of.
From www.kolabtree.com
Medical Device Design Le guide essentiel, étape par étape Medical Device Design Input Examples With this guide, i plan to. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are essential for medical device development. Needs and expectations of business, clinical, and user. Medical Device Design Input Examples.
From www.youtube.com
Design Control for Medical Devices Online introductory course YouTube Medical Device Design Input Examples Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. With this guide, i plan to. Design inputs are the foundation of a medical device, and your. Medical Device Design Input Examples.
From c3mdc.com
User Needs & Design Input Requirements C3 Medical Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Well established design inputs can make the rest of. In this example, design verification checks that your. Medical Device Design Input Examples.
From www.joharidigital.com
What is Design History File? Why it is Important for Medical Device Medical Device Design Input Examples Needs and expectations of business, clinical, and user stakeholders. With this guide, i plan to. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Well established design inputs can make the rest of. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just. Medical Device Design Input Examples.
From www.modernrequirements.com
Facilitate the Medical Device Design Controls Modern Requirements Medical Device Design Input Examples Needs and expectations of business, clinical, and user stakeholders. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Design inputs are supposed to be objective criteria for verification that the design outputs are. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing. Medical Device Design Input Examples.
From www.jamasoftware.com
Design Inputs in Jama Connect for Medical Device Development Jama Medical Device Design Input Examples Let’s look at a basic example: Well established design inputs can make the rest of. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Safe medical device act of 1990 authorized fda to add design. Medical Device Design Input Examples.
From www.alecalpert.com
How to Make Design Controls More Efficient With Traceability Matrix Medical Device Design Input Examples With this guide, i plan to. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Needs and expectations of business, clinical, and user stakeholders. Let’s look at. Medical Device Design Input Examples.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Design Input Examples Let’s look at a basic example: Well established design inputs can make the rest of. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are essential for medical device development. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash. Medical Device Design Input Examples.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Design Input Examples Let’s look at a basic example: In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Say your input is “the length of the device should be between. Medical Device Design Input Examples.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device Design Input Examples Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Let’s look at a basic example: Well established design inputs can make the rest of. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Needs and expectations of business, clinical, and user stakeholders. In. Medical Device Design Input Examples.
From innolitics.com
Design Inputs Best Practices, FAQs, and Examples Medical Device Design Input Examples Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Let’s look at a basic example:. Medical Device Design Input Examples.
From www.youtube.com
User Needs and Design Inputs YouTube Medical Device Design Input Examples Design inputs are supposed to be objective criteria for verification that the design outputs are. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. In this example, design verification checks that. Medical Device Design Input Examples.
From www.orielstat.com
Basics of Medical Device Design Controls What, Why, and How Oriel Medical Device Design Input Examples Design inputs are supposed to be objective criteria for verification that the design outputs are. Let’s look at a basic example: In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used. Medical Device Design Input Examples.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device Design Input Examples Well established design inputs can make the rest of. Design inputs are supposed to be objective criteria for verification that the design outputs are. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Design inputs are essential for medical device development. With this guide, i plan. Medical Device Design Input Examples.
From www.pinterest.co.uk
Explore Our Image of Medical Device Design And Development Plan Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. With this guide, i plan to. Well established design inputs can make the rest of. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Say your. Medical Device Design Input Examples.
From operonstrategist.com
FDA Design Control The Ultimate Guide For Medical Device Companies Medical Device Design Input Examples Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Needs and expectations of business, clinical, and user stakeholders. With this guide, i plan to. Design inputs are essential for medical device development. Design inputs are the foundation of a medical device, and your device is only. Medical Device Design Input Examples.
From issuu.com
Design Inputs Template by Pharmi Med Ltd Issuu Medical Device Design Input Examples Design inputs are essential for medical device development. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Needs and expectations of business, clinical, and user stakeholders. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Say. Medical Device Design Input Examples.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. With this guide, i plan to. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet. Medical Device Design Input Examples.
From www.pinterest.com
Browse Our Sample of Medical Device Design And Development Plan Medical Device Design Input Examples Needs and expectations of business, clinical, and user stakeholders. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. With this guide, i plan to. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Design inputs are essential for medical device development. In this. Medical Device Design Input Examples.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Medical Device Design Input Examples Design inputs are supposed to be objective criteria for verification that the design outputs are. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Let’s look at a basic example: In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs.. Medical Device Design Input Examples.
From www.orielstat.com
Design Input & Output Medical Devices Oriel STAT A MATRIX Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Needs and expectations of business, clinical, and user stakeholders. Design inputs are supposed to be objective criteria. Medical Device Design Input Examples.
From www.joharidigital.com
Medical Device Design and Development The Ultimate Guide from scratch Medical Device Design Input Examples Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Needs and expectations of business, clinical, and user stakeholders. Let’s look at a basic example: With this guide, i plan to. Design inputs are supposed to be objective criteria for verification that the design outputs are. In. Medical Device Design Input Examples.
From www.greenlight.guru
Design Controls For Medical Device Companies [Guide] Medical Device Design Input Examples Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Let’s look at a basic example: Safe medical device act of 1990 authorized fda to add design controls to the current good. Medical Device Design Input Examples.
From www.greenlight.guru
How to Translate Voice of Customer into User Needs Medical Device Design Input Examples Needs and expectations of business, clinical, and user stakeholders. With this guide, i plan to. Let’s look at a basic example: Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are the. Medical Device Design Input Examples.
From www.researchgate.net
(PDF) Identifying the initial specification of Design Inputs & Outputs Medical Device Design Input Examples Well established design inputs can make the rest of. Design inputs are essential for medical device development. Let’s look at a basic example: Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. With this guide, i plan to. Design inputs are supposed to be objective criteria. Medical Device Design Input Examples.
From www.orielstat.com
Basics FDA Medical Design Controls Oriel STAT A MATRIX Medical Device Design Input Examples In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Design inputs are essential for medical device development. Say your input is “the length of the device should be between 5 and 10 cm.” the mistake is to just rehash this. With this guide, i plan to. Design controls demonstrate our. Medical Device Design Input Examples.
From www.greenlight.guru
The Art of Defining Design Inputs And Design Outputs Medical Device Design Input Examples Let’s look at a basic example: Design inputs are supposed to be objective criteria for verification that the design outputs are. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Well established design inputs can make the rest of. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing. Medical Device Design Input Examples.
From www.johner-institut.de
Design Input und Output Definition und Requirements Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Design inputs are essential for medical device development. With this guide, i plan to. Well established design inputs can make the rest of. In this example, design verification checks that your design outputs—such as drawings, specifications, or. Medical Device Design Input Examples.
From tsqasia.com
Medical Devices EU Design Control Process Support TSQ Middle East & Asia Medical Device Design Input Examples Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. With this guide, i plan to. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Say your input is “the length of the device should be between 5 and 10 cm.”. Medical Device Design Input Examples.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development Medical Device Design Input Examples Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are essential for medical device development. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Let’s look at a basic example: Say your input is “the length of the. Medical Device Design Input Examples.
From lettertheme.blogspot.com
Medical Device Design And Development Plan Template Medical Device Design Input Examples Design inputs are essential for medical device development. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs. Needs and expectations of business, clinical, and user stakeholders. With this guide, i. Medical Device Design Input Examples.
From synergio.nl
Agile Medical Device Development Do or Don't? Content aspects Medical Device Design Input Examples Well established design inputs can make the rest of. Design inputs are supposed to be objective criteria for verification that the design outputs are. Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Say your input is “the length of the device should be between 5 and 10 cm.”. Medical Device Design Input Examples.
From old.sermitsiaq.ag
Medical Device Traceability Matrix Template Medical Device Design Input Examples Safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp) requirements. Let’s look at a basic example: Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. With this guide, i plan to. Design inputs are essential for. Medical Device Design Input Examples.
From www.qualitymeddev.com
IEC 623042006 Medical Device Software QualityMedDev Medical Device Design Input Examples Design inputs are essential for medical device development. Needs and expectations of business, clinical, and user stakeholders. Design inputs are the foundation of a medical device, and your device is only as effective as the inputs used to create it. Let’s look at a basic example: Well established design inputs can make the rest of. With this guide, i plan. Medical Device Design Input Examples.
From dxohuofkv.blob.core.windows.net
Input Process Output Examples Computer at Charles Lubin blog Medical Device Design Input Examples With this guide, i plan to. Design controls demonstrate our medical devices are safe, effective, and meet the indications for use. Design inputs are supposed to be objective criteria for verification that the design outputs are. Design inputs are essential for medical device development. Design inputs are the foundation of a medical device, and your device is only as effective. Medical Device Design Input Examples.