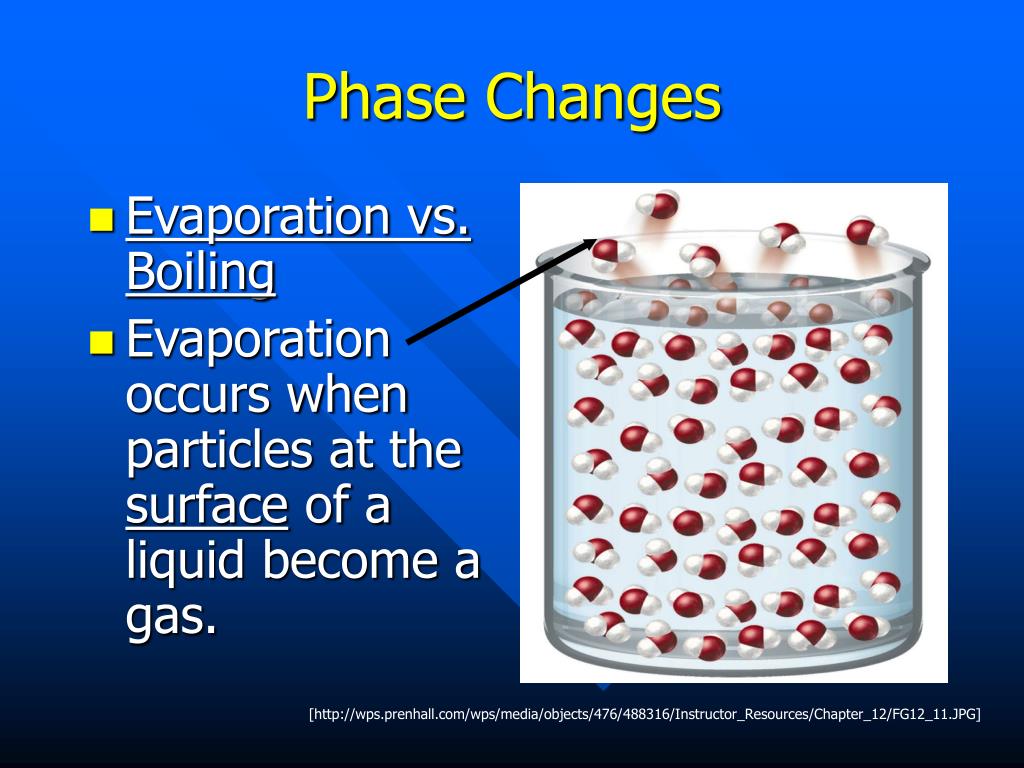
Do You Think That The Pressure Will Affect The Phase Changes . These changes occur when sufficient. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. from our free online course, “science & cooking: Pressure can also be used to change the phase of the substance. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. For instance, at atmospheric pressure, water melts at 0 °c and. In the picture above, we have a. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing.
from www.slideserve.com
a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. from our free online course, “science & cooking: In the picture above, we have a. For instance, at atmospheric pressure, water melts at 0 °c and. These changes occur when sufficient. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. Pressure can also be used to change the phase of the substance.
PPT Phase Changes PowerPoint Presentation, free download ID5049190
Do You Think That The Pressure Will Affect The Phase Changes from our free online course, “science & cooking: For instance, at atmospheric pressure, water melts at 0 °c and. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. from our free online course, “science & cooking: a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. These changes occur when sufficient. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. Pressure can also be used to change the phase of the substance.
From www.youtube.com
What is the effect of pressure change on equilibrium Class 10 Do You Think That The Pressure Will Affect The Phase Changes note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. For instance, at atmospheric pressure, water melts at 0 °c and. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. from our free online course, “science & cooking: These changes. Do You Think That The Pressure Will Affect The Phase Changes.
From socratic.org
What is the relation between critical temperature and boiling point or Do You Think That The Pressure Will Affect The Phase Changes from our free online course, “science & cooking: In the picture above, we have a. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. . Do You Think That The Pressure Will Affect The Phase Changes.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Do You Think That The Pressure Will Affect The Phase Changes note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some. Do You Think That The Pressure Will Affect The Phase Changes.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Do You Think That The Pressure Will Affect The Phase Changes For instance, at atmospheric pressure, water melts at 0 °c and. Pressure can also be used to change the phase of the substance. In the picture above, we have a. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. These changes occur when sufficient. atmospheric. Do You Think That The Pressure Will Affect The Phase Changes.
From nursehub.com
Phase Changes NurseHub Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. from our free online course, “science & cooking: note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. In the picture above, we have a. For instance, at atmospheric pressure, water. Do You Think That The Pressure Will Affect The Phase Changes.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. These changes occur when sufficient. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. from our free online course, “science &. Do You Think That The Pressure Will Affect The Phase Changes.
From www.101diagrams.com
Phase Change Diagrams 101 Diagrams Do You Think That The Pressure Will Affect The Phase Changes atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. from our free online course, “science & cooking: during a phase transition of a given medium certain properties of the medium change, often. Do You Think That The Pressure Will Affect The Phase Changes.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Do You Think That The Pressure Will Affect The Phase Changes note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. from our free online course, “science & cooking: For instance, at atmospheric pressure, water melts at. Do You Think That The Pressure Will Affect The Phase Changes.
From lessonlibrarymildew.z21.web.core.windows.net
Diagram Of Phase Changes Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. These changes occur when sufficient. For instance, at atmospheric pressure, water melts at 0 °c and. In the picture above, we have a. during. Do You Think That The Pressure Will Affect The Phase Changes.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 Do You Think That The Pressure Will Affect The Phase Changes These changes occur when sufficient. For instance, at atmospheric pressure, water melts at 0 °c and. In the picture above, we have a. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by. Do You Think That The Pressure Will Affect The Phase Changes.
From slideplayer.com
Topics in CHM 1046 Intermolecular forces (IMF) Themodynamics ppt download Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. These changes occur when sufficient. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. Pressure can also be used to change the phase of the substance. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two. Do You Think That The Pressure Will Affect The Phase Changes.
From circuitaryns.z14.web.core.windows.net
How To Read A Phase Change Diagram Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. from our free online course, “science & cooking: These changes occur when sufficient. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. For instance, at atmospheric pressure, water melts at 0 °c and.. Do You Think That The Pressure Will Affect The Phase Changes.
From www.reddit.com
What is the effect of air pressure on melting point of water? r Do You Think That The Pressure Will Affect The Phase Changes These changes occur when sufficient. For instance, at atmospheric pressure, water melts at 0 °c and. from our free online course, “science & cooking: Pressure can also be used to change the phase of the substance. In the picture above, we have a. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two. Do You Think That The Pressure Will Affect The Phase Changes.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. For instance, at atmospheric pressure, water melts at 0 °c and. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. In the picture above, we have a. These. Do You Think That The Pressure Will Affect The Phase Changes.
From opentextbc.ca
Phase Changes Basic HVAC Do You Think That The Pressure Will Affect The Phase Changes during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. For instance, at atmospheric pressure, water melts at 0 °c and. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. These changes occur when sufficient. from our free. Do You Think That The Pressure Will Affect The Phase Changes.
From chem.libretexts.org
Fundamentals of Phase Transitions Chemistry LibreTexts Do You Think That The Pressure Will Affect The Phase Changes Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. These changes occur when sufficient. atmospheric pressure will not. Do You Think That The Pressure Will Affect The Phase Changes.
From www.youtube.com
The Effect of Pressure Changes on Equilibrium YouTube Do You Think That The Pressure Will Affect The Phase Changes during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. These changes occur when sufficient. In the picture above, we have a. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. atmospheric pressure will not affect intermolecular. Do You Think That The Pressure Will Affect The Phase Changes.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry Do You Think That The Pressure Will Affect The Phase Changes Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. from our free online course, “science & cooking: These changes occur when sufficient. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and. Do You Think That The Pressure Will Affect The Phase Changes.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a. from our free online course, “science & cooking: Pressure can also be used to change the phase of the substance. note that, at a fixed temperature, you can change the phase from solid. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Phase Changes & Phase Diagrams PowerPoint Presentation, free Do You Think That The Pressure Will Affect The Phase Changes These changes occur when sufficient. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. note that, at a fixed temperature, you can change the phase from solid. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Do You Think That The Pressure Will Affect The Phase Changes Pressure can also be used to change the phase of the substance. For instance, at atmospheric pressure, water melts at 0 °c and. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. In the picture above, we have a. These changes occur when sufficient. atmospheric pressure will not affect intermolecular. Do You Think That The Pressure Will Affect The Phase Changes.
From courses.lumenlearning.com
Phase Changes Physics Do You Think That The Pressure Will Affect The Phase Changes Pressure can also be used to change the phase of the substance. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. from our free online course, “science & cooking: note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. These changes. Do You Think That The Pressure Will Affect The Phase Changes.
From sciencenotes.org
States of Matter Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. These changes occur when sufficient. during a phase transition of a given medium certain properties of the. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. For instance, at atmospheric pressure, water melts at 0 °c and. Pressure. Do You Think That The Pressure Will Affect The Phase Changes.
From philschatz.com
Phase Changes · Physics Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. Pressure can also be used to change the phase of the substance. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. These. Do You Think That The Pressure Will Affect The Phase Changes.
From www.coursehero.com
Solid to Gas Phase Transition Introduction to Chemistry Course Hero Do You Think That The Pressure Will Affect The Phase Changes atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. from our free online course, “science & cooking: during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. note that, at a fixed temperature, you can change the. Do You Think That The Pressure Will Affect The Phase Changes.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula Do You Think That The Pressure Will Affect The Phase Changes These changes occur when sufficient. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. Pressure can also be used to change the phase of the substance. from our free online. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Do You Think That The Pressure Will Affect The Phase Changes a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. during a phase transition. Do You Think That The Pressure Will Affect The Phase Changes.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Do You Think That The Pressure Will Affect The Phase Changes These changes occur when sufficient. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. from our free online course, “science & cooking: Pressure can also be used. Do You Think That The Pressure Will Affect The Phase Changes.
From www.youtube.com
Pressure and Velocity Changes Through an Expansion (Interactive) YouTube Do You Think That The Pressure Will Affect The Phase Changes In the picture above, we have a. during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. These changes occur when sufficient. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature,. Do You Think That The Pressure Will Affect The Phase Changes.
From schematictimiwottobe7y.z22.web.core.windows.net
What Is A Phase Diagram Apex Do You Think That The Pressure Will Affect The Phase Changes For instance, at atmospheric pressure, water melts at 0 °c and. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. during a phase transition of a given medium certain properties of the medium. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Do You Think That The Pressure Will Affect The Phase Changes note that, at a fixed temperature, you can change the phase from solid (ice) to liquid (water) by increasing. In the picture above, we have a. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. a phase change is when matter changes to from one state (solid, liquid, gas, plasma). Do You Think That The Pressure Will Affect The Phase Changes.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Do You Think That The Pressure Will Affect The Phase Changes Pressure can also be used to change the phase of the substance. In the picture above, we have a. from our free online course, “science & cooking: For instance, at atmospheric pressure, water melts at 0 °c and. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. during a. Do You Think That The Pressure Will Affect The Phase Changes.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Do You Think That The Pressure Will Affect The Phase Changes during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. In the picture above, we have a. a phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. note that, at a fixed temperature, you can change the phase. Do You Think That The Pressure Will Affect The Phase Changes.
From www.thoughtco.com
Phase Diagrams Phases of Matter and Phase Transitions Do You Think That The Pressure Will Affect The Phase Changes during a phase transition of a given medium certain properties of the medium change, often discontinuously, as a result of some external. from our free online course, “science & cooking: In the picture above, we have a. atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase. a phase change. Do You Think That The Pressure Will Affect The Phase Changes.