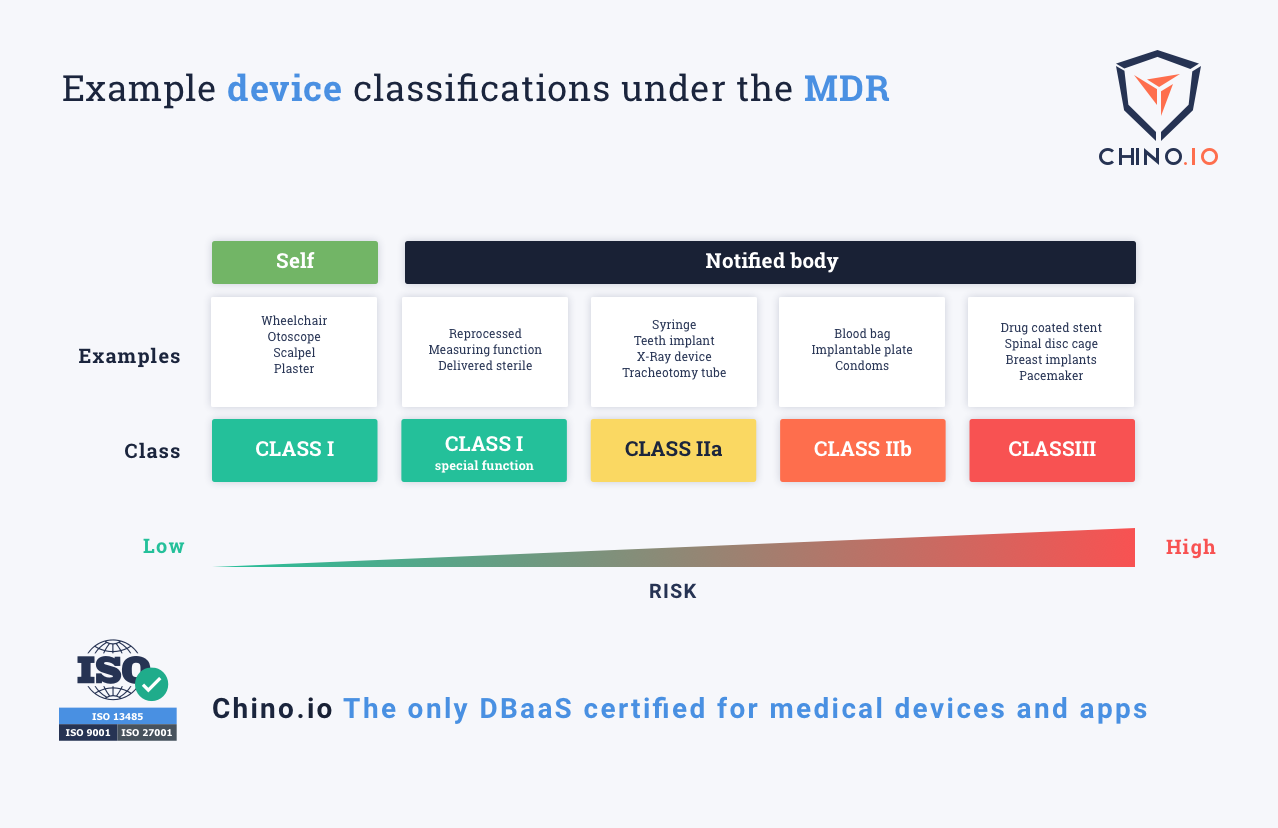
Medical Device Class Iia Definition . Mdr class iib medical devices contain contact lenses, surgical lasers, and. What is a class 2 medical device? Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia medical devices pose a medium risk to. In turn, this determines certain requirements, and which conformity assessment procedure the. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. This means that unlike a class i device, the manufacturer must.
from blog.chino.io
Mdr class iib medical devices contain contact lenses, surgical lasers, and. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. More specifically, medical devices are defined as class i, iia, iib, or iii. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Class iia medical devices pose a medium risk to. In turn, this determines certain requirements, and which conformity assessment procedure the. This means that unlike a class i device, the manufacturer must. What is a class 2 medical device?
What MDR class is my eHealth app?
Medical Device Class Iia Definition More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Mdr class iib medical devices contain contact lenses, surgical lasers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. This means that unlike a class i device, the manufacturer must. In turn, this determines certain requirements, and which conformity assessment procedure the. What is a class 2 medical device? More specifically, medical devices are defined as class i, iia, iib, or iii. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia medical devices pose a medium risk to.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia medical devices pose a medium risk to. This means that unlike a class i device, the manufacturer must. Mdr class iib. Medical Device Class Iia Definition.
From www.pjurlove.com
Medical Device vs. Cosmetic Product Know the Difference Medical Device Class Iia Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Class iia medical devices pose a medium risk to. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in. Medical Device Class Iia Definition.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Class Iia Definition Class iia medical devices pose a medium risk to. More specifically, medical devices are defined as class i, iia, iib, or iii. Mdr class iib medical devices contain contact lenses, surgical lasers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In turn, this determines certain requirements, and. Medical Device Class Iia Definition.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Class Iia Definition Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Class iia medical devices pose a medium risk to. What is a class 2 medical device? In turn, this determines certain requirements, and which conformity assessment procedure the. Mdr class iib medical devices contain contact lenses, surgical lasers, and.. Medical Device Class Iia Definition.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 Medical Device Class Iia Definition This means that unlike a class i device, the manufacturer must. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Mdr class iib medical. Medical Device Class Iia Definition.
From decomplix.com
Medical device software (MDSW) under EU MDR and IVDR Medical Device Class Iia Definition In turn, this determines certain requirements, and which conformity assessment procedure the. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Regulation (eu). Medical Device Class Iia Definition.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Class Iia Definition More specifically, medical devices are defined as class i, iia, iib, or iii. This means that unlike a class i device, the manufacturer must. What is a class 2 medical device? Mdr class iib medical devices contain contact lenses, surgical lasers, and. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices,. Medical Device Class Iia Definition.
From www.slideserve.com
PPT Does All Medical Devices Needing a CE Mark PowerPoint Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Class iia medical devices pose a medium risk to. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a. Medical Device Class Iia Definition.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Medical Device Class Iia Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Class iia medical devices pose a medium risk to. More specifically, medical devices are defined as class i, iia, iib, or iii. What is a class 2 medical device? In turn, this determines certain requirements, and which conformity assessment procedure. Medical Device Class Iia Definition.
From blog.chino.io
What MDR class is my eHealth app? Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. What is a class 2 medical device? Class iia medical devices pose a medium risk to. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. More specifically, medical devices are defined as class i, iia, iib, or iii.. Medical Device Class Iia Definition.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Class Iia Definition This means that unlike a class i device, the manufacturer must. Mdr class iib medical devices contain contact lenses, surgical lasers, and. In turn, this determines certain requirements, and which conformity assessment procedure the. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. More. Medical Device Class Iia Definition.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Medical Device Class Iia Definition Class iia medical devices pose a medium risk to. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. More specifically, medical devices are defined as class i, iia, iib, or iii. In turn, this determines certain requirements, and which conformity assessment procedure the. Mdr. Medical Device Class Iia Definition.
From www.arenasolutions.com
Class II Device Definition Arena Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. More specifically, medical devices are defined as class i, iia, iib, or iii. This means that unlike a class i device, the manufacturer must. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. In turn, this determines certain requirements, and which conformity assessment procedure the. Regulation. Medical Device Class Iia Definition.
From mungfali.com
Classification Of Medical Devices Medical Device Class Iia Definition Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Class iia devices generally include low to medium risk and refer mainly to devices installed. Medical Device Class Iia Definition.
From loesbuehk.blob.core.windows.net
Medical Device User Needs Examples at Rebecca Barrow blog Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Class iia medical devices pose a medium risk to. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. This means that unlike a class i device, the manufacturer must. What is a. Medical Device Class Iia Definition.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. In turn, this determines certain requirements, and which conformity assessment procedure the. What is a class 2 medical device? Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on. Medical Device Class Iia Definition.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. This means that unlike a class i device, the manufacturer must. What is a class 2 medical device? Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia devices generally include. Medical Device Class Iia Definition.
From vem-medical.com
Medical Device Manufacturing Medical Device Class Iia Definition Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. This means that unlike a class i device, the manufacturer must. What is a class 2 medical device? Mdr class iib medical devices contain contact lenses, surgical lasers, and. In turn, this determines certain requirements, and which conformity assessment procedure the. To get a basic understanding of what. Medical Device Class Iia Definition.
From slideplayer.com
EU Medical Devices Regulatory System WHO Forum India December 2018 Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Mdr class iib medical devices contain contact lenses, surgical lasers, and. Class iia devices generally. Medical Device Class Iia Definition.
From joiezthhz.blob.core.windows.net
Standard Medical Device Regulation at Moises Cox blog Medical Device Class Iia Definition This means that unlike a class i device, the manufacturer must. Mdr class iib medical devices contain contact lenses, surgical lasers, and. Class iia medical devices pose a medium risk to. What is a class 2 medical device? Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Class iia. Medical Device Class Iia Definition.
From joikszgey.blob.core.windows.net
What Are The Fda Medical Device Classifications at Roy Metcalf blog Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. More specifically, medical devices are defined as class i, iia, iib, or iii. What is a. Medical Device Class Iia Definition.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Class Iia Definition What is a class 2 medical device? Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Class iia medical devices pose a medium risk to. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each. Medical Device Class Iia Definition.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Class Iia Definition More specifically, medical devices are defined as class i, iia, iib, or iii. What is a class 2 medical device? Mdr class iib medical devices contain contact lenses, surgical lasers, and. In turn, this determines certain requirements, and which conformity assessment procedure the. This means that unlike a class i device, the manufacturer must. Mdr class iia medical devices contain. Medical Device Class Iia Definition.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Class Iia Definition Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia medical devices pose a medium risk to. Mdr class iib medical devices contain contact lenses, surgical lasers, and. Regulation (eu) 2017/745 of the european. Medical Device Class Iia Definition.
From www.ce-mark.in
CE认证网 IIa 类医疗器械的CE认证途径 at Medical Device Class Iia Definition This means that unlike a class i device, the manufacturer must. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is a class 2 medical device? More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia medical devices pose a medium risk to.. Medical Device Class Iia Definition.
From simplerqms.com
EU MDR Medical Device Classification Classes and Examples Medical Device Class Iia Definition Class iia medical devices pose a medium risk to. Mdr class iib medical devices contain contact lenses, surgical lasers, and. More specifically, medical devices are defined as class i, iia, iib, or iii. What is a class 2 medical device? Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the. Medical Device Class Iia Definition.
From kladuvsja.blob.core.windows.net
Medical Device Regulation Eu at Hay blog Medical Device Class Iia Definition In turn, this determines certain requirements, and which conformity assessment procedure the. What is a class 2 medical device? Class iia medical devices pose a medium risk to. More specifically, medical devices are defined as class i, iia, iib, or iii. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. This means that unlike a class i. Medical Device Class Iia Definition.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Class Iia Definition To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. Class iia medical devices pose a medium risk to. More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia devices generally include low to medium risk and refer mainly to. Medical Device Class Iia Definition.
From www.pjurlove.com
pjur love more consumer safety The pjur group launches certified class Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. This means that unlike a class i device, the manufacturer must. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. In turn, this determines certain requirements, and which conformity assessment procedure the. More. Medical Device Class Iia Definition.
From loeolkifl.blob.core.windows.net
What Is A Medical Device Eu at Jonathan Eady blog Medical Device Class Iia Definition This means that unlike a class i device, the manufacturer must. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Class iia medical devices pose a medium risk to. To get a basic understanding of what. Medical Device Class Iia Definition.
From www.fairprice.com.sg
Medel Ear Temp Infrared Thermometer, Class IIa Medical Device NTUC Medical Device Class Iia Definition Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. More specifically, medical devices are defined as class i, iia, iib, or iii. Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april. Medical Device Class Iia Definition.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Medical Device Class Iia Definition In turn, this determines certain requirements, and which conformity assessment procedure the. This means that unlike a class i device, the manufacturer must. What is a class 2 medical device? Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. To get a basic understanding of what classes each regulatory. Medical Device Class Iia Definition.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Medical Device Class Iia Definition More specifically, medical devices are defined as class i, iia, iib, or iii. What is a class 2 medical device? Class iia devices generally include low to medium risk and refer mainly to devices installed within the body in the short. In turn, this determines certain requirements, and which conformity assessment procedure the. Regulation (eu) 2017/745 of the european parliament. Medical Device Class Iia Definition.
From exymbnavg.blob.core.windows.net
Medical Devices And Equipment Mean at Sheila Washburn blog Medical Device Class Iia Definition Mdr class iib medical devices contain contact lenses, surgical lasers, and. What is a class 2 medical device? More specifically, medical devices are defined as class i, iia, iib, or iii. In turn, this determines certain requirements, and which conformity assessment procedure the. This means that unlike a class i device, the manufacturer must. Class iia devices generally include low. Medical Device Class Iia Definition.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Medical Device Class Iia Definition Mdr class iia medical devices contain catheters, hearing aids, and surgical clamps. Mdr class iib medical devices contain contact lenses, surgical lasers, and. To get a basic understanding of what classes each regulatory organization has and what they mean, let’s take a brief look at each of them. This means that unlike a class i device, the manufacturer must. Regulation. Medical Device Class Iia Definition.