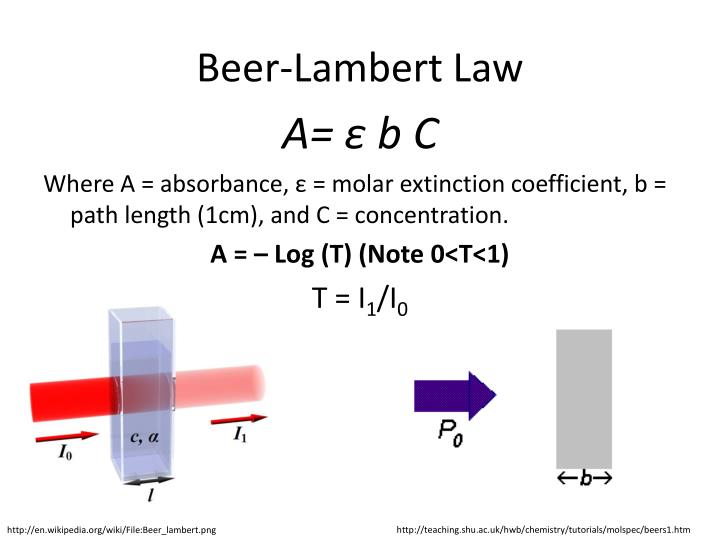
From www.slideserve.com
PPT SPECTROPHOTOMETRY PowerPoint Presentation, free download ID5761859 Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From www.researchgate.net
Application of the BeerLambert law to blue and NIR light Comparison of Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Beer Lambert Law Concentration YouTube Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.slideserve.com
PPT Protein Concentration Determination PowerPoint Presentation ID Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.coursehero.com
[Solved] What is the LambertBeer Law and how can you use it to Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer Lambert Law Rearranged For Concentration.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Rearranged For Concentration Use the information in the problem statement to determine what variables are given and. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From chemistrypubs.com
BeerLambert Law Equation, Derivation, & Uses Chemistrupubs Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From study.com
Using the BeerLambert Law to Calculate the Concentration of a Solution Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Set up the equation of the line to show the relation between absorbance and concentration. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
B.7 The Beer Lambert law (HL) YouTube Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Rearranged For Concentration Set up the equation of the line to show the relation between absorbance and concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Beer Lambert Law Rearranged For Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Set up the equation of the line to show the relation between absorbance and concentration. Use the information in the problem statement to determine what variables are given and. Beer Lambert Law Rearranged For Concentration.