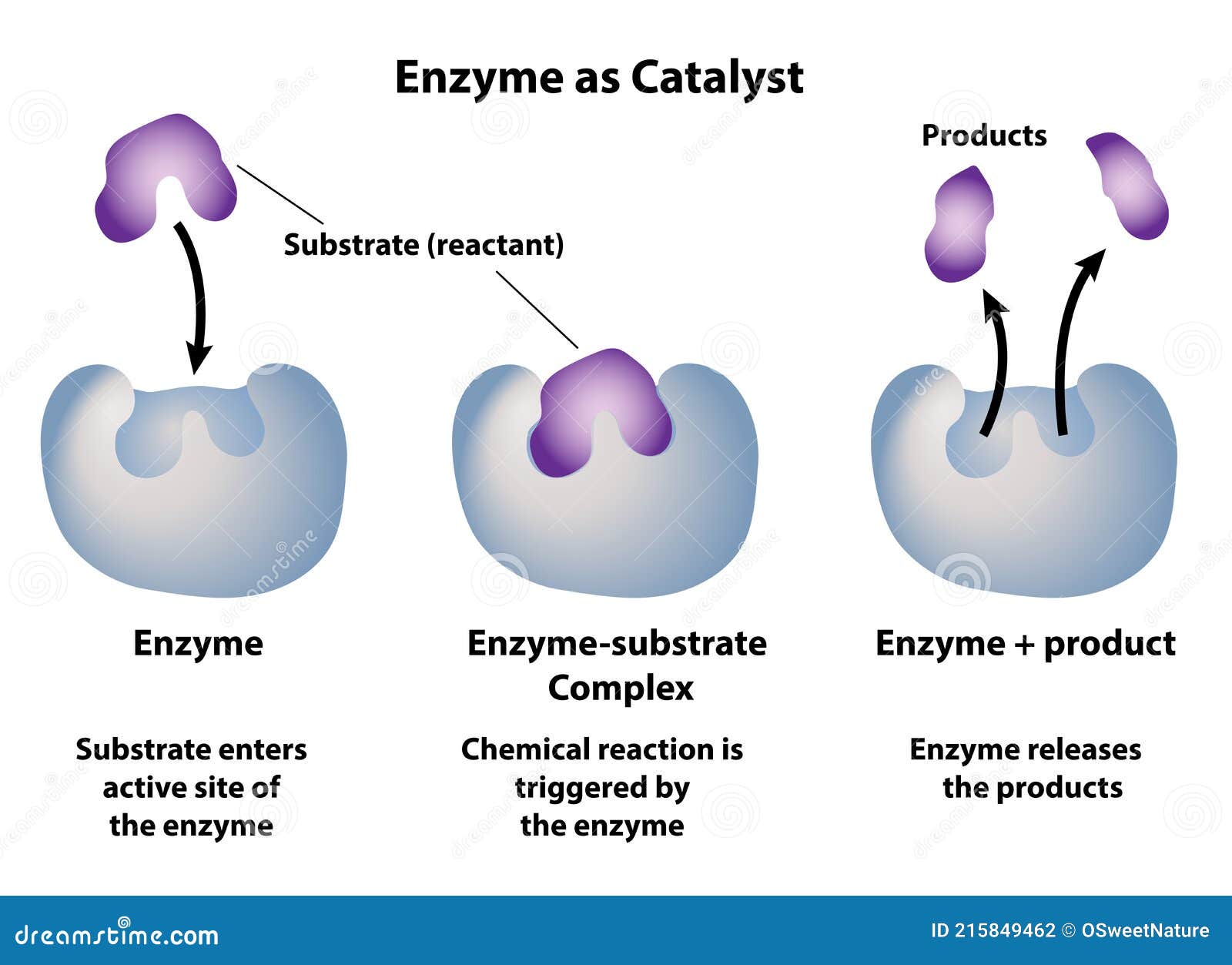
Enzymatic Catalysis Hydrolysis . The molecular basis of enzymatic catalysis. In contrast, enzymes are much more specific. This example illustrates several features of enzymatic catalysis; Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis).
from www.dreamstime.com
For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. This example illustrates several features of enzymatic catalysis; An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). Catalysis is a process that increases the rate at which a reaction approaches equilibrium.
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of
Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. This example illustrates several features of enzymatic catalysis; Catalysis is a process that increases the rate at which a reaction approaches equilibrium. The molecular basis of enzymatic catalysis. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. In contrast, enzymes are much more specific.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID590166 Enzymatic Catalysis Hydrolysis An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only. Enzymatic Catalysis Hydrolysis.
From www.researchgate.net
Mechanism of the hydrolysis reaction of ester bonds catalyzed by Enzymatic Catalysis Hydrolysis For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. This example illustrates several features of enzymatic catalysis; In contrast, enzymes are much more specific. Many enzymes have active site serines which act as. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Mechanisms of Enzyme Action PowerPoint Presentation ID2019809 Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Applied Enzyme Catalysis PowerPoint Presentation, free download Enzymatic Catalysis Hydrolysis Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. This example illustrates several features of enzymatic catalysis; The molecular basis. Enzymatic Catalysis Hydrolysis.
From silab.fr
Focus on enzymatic hydrolysis SILAB Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. This example illustrates several features of enzymatic catalysis; For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis. Enzymatic Catalysis Hydrolysis.
From www.researchgate.net
Two main modes of enzymecatalyzed hydrolysis of Nacylhomoserine Enzymatic Catalysis Hydrolysis This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Endergonic and Exergonic Reactions PowerPoint Presentation, free Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. In contrast, enzymes are much more specific. This example illustrates several features of enzymatic catalysis; An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. Many enzymes have active site serines which. Enzymatic Catalysis Hydrolysis.
From studylib.net
Hydrolysis Enzymatic Catalysis Hydrolysis For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). Catalysis is a process that increases the rate. Enzymatic Catalysis Hydrolysis.
From zymvol.com
All you need to know about enzymes Zymvol Enzymatic Catalysis Hydrolysis For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of. Enzymatic Catalysis Hydrolysis.
From www.researchgate.net
Scheme 4. Cellulose enzymatic hydrolysis mechanism. Download Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. The molecular basis of enzymatic catalysis. This example illustrates several features of enzymatic catalysis; An inorganic acid. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Applied Enzyme Catalysis PowerPoint Presentation, free download Enzymatic Catalysis Hydrolysis This example illustrates several features of enzymatic catalysis; Catalysis is a process that increases the rate at which a reaction approaches equilibrium. The molecular basis of enzymatic catalysis. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality.. Enzymatic Catalysis Hydrolysis.
From quizlet.com
Figure 8.8 Enzymecatalyzed synthesis and hydrolysis reactions Diagram Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. For a kinetically perfect enzyme, every encounter. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzyme CatalysisSerine Proteases PowerPoint Presentation, free Enzymatic Catalysis Hydrolysis Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. For a kinetically perfect enzyme, every encounter between enzyme and substrate. Enzymatic Catalysis Hydrolysis.
From www.chegg.com
Solved The enzyme catalyzed hydrolysis mechanism of RNA is Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT CHAPTER 6 Enzymes PowerPoint Presentation, free download ID815181 Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Chapter 14 Mechanisms of Enzyme Action PowerPoint Presentation Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. Many enzymes have active site serines which. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID2961222 Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. The molecular basis of enzymatic catalysis. This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). For a kinetically perfect enzyme, every encounter between. Enzymatic Catalysis Hydrolysis.
From www.numerade.com
SOLVED This diagram shows the catalytic hydrolysis of sucrose into the Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. The molecular basis of enzymatic catalysis. In contrast, enzymes are much more specific. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction rates of. Enzymatic Catalysis Hydrolysis.
From www.thoughtco.com
An Explanation of the Process Hydrolysis Enzymatic Catalysis Hydrolysis This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT The Organic Chemistry of EnzymeCatalyzed Reactions Revised Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. For a kinetically perfect enzyme, every encounter. Enzymatic Catalysis Hydrolysis.
From silab.fr
Focus on enzymatic hydrolysis SILAB Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. The molecular basis of enzymatic catalysis. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different. Enzymatic Catalysis Hydrolysis.
From www.pinterest.com
Catalyst and Mechanism of Enzyme Catalysis Enzymes, Chemistry, Active Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. This example illustrates several features of enzymatic catalysis; In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides,. Enzymatic Catalysis Hydrolysis.
From www.researchgate.net
Mechanisms of ester hydrolysis under acid or base catalysis. Download Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. In contrast, enzymes are much more specific. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. An inorganic acid such as sulfuric acid can be used to. Enzymatic Catalysis Hydrolysis.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. This example illustrates several features of enzymatic catalysis; Catalysis is a process that increases the rate at which a reaction approaches equilibrium. In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides,. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID226444 Enzymatic Catalysis Hydrolysis In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. The molecular basis of enzymatic catalysis. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. For. Enzymatic Catalysis Hydrolysis.
From www.semanticscholar.org
Figure 1 from Hydrolysis of LArginine Chemical and Enzymatic Enzymatic Catalysis Hydrolysis An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). The molecular basis of. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID226444 Enzymatic Catalysis Hydrolysis An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). Catalysis is a. Enzymatic Catalysis Hydrolysis.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Enzymatic Catalysis Hydrolysis An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic. Enzymatic Catalysis Hydrolysis.
From www.researchgate.net
Mechanism of ACh hydrolysis catalyzed by AChE. Download Scientific Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. In contrast, enzymes are much more specific. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can. Enzymatic Catalysis Hydrolysis.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Enzymatic Catalysis Hydrolysis This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited.. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Enzymatic Catalysis Hydrolysis The molecular basis of enzymatic catalysis. In contrast, enzymes are much more specific. An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. Catalysis is a process that increases the rate at which a reaction approaches equilibrium. This. Enzymatic Catalysis Hydrolysis.
From chem.libretexts.org
5.3 Mechanism of Enzymatic Catalysis Chemistry LibreTexts Enzymatic Catalysis Hydrolysis For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. In contrast, enzymes are much more specific. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction. Enzymatic Catalysis Hydrolysis.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Enzymatic Catalysis Hydrolysis Catalysis is a process that increases the rate at which a reaction approaches equilibrium. For a kinetically perfect enzyme, every encounter between enzyme and substrate leads to product and hence the reaction velocity is only limited. Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid. Enzymatic Catalysis Hydrolysis.
From www.bio1152.nicerweb.com
enzyme.html 05_16EnzymeCatalyticCycle.jpg Enzymatic Catalysis Hydrolysis Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. This example illustrates several features of enzymatic catalysis; Catalysis is a. Enzymatic Catalysis Hydrolysis.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID3359630 Enzymatic Catalysis Hydrolysis This example illustrates several features of enzymatic catalysis; Many enzymes have active site serines which act as nucleophilic catalysts in nucleophilic substitution reactions (usually hydrolysis). Catalysis is a process that increases the rate at which a reaction approaches equilibrium. In contrast, enzymes are much more specific. The molecular basis of enzymatic catalysis. For a kinetically perfect enzyme, every encounter between. Enzymatic Catalysis Hydrolysis.