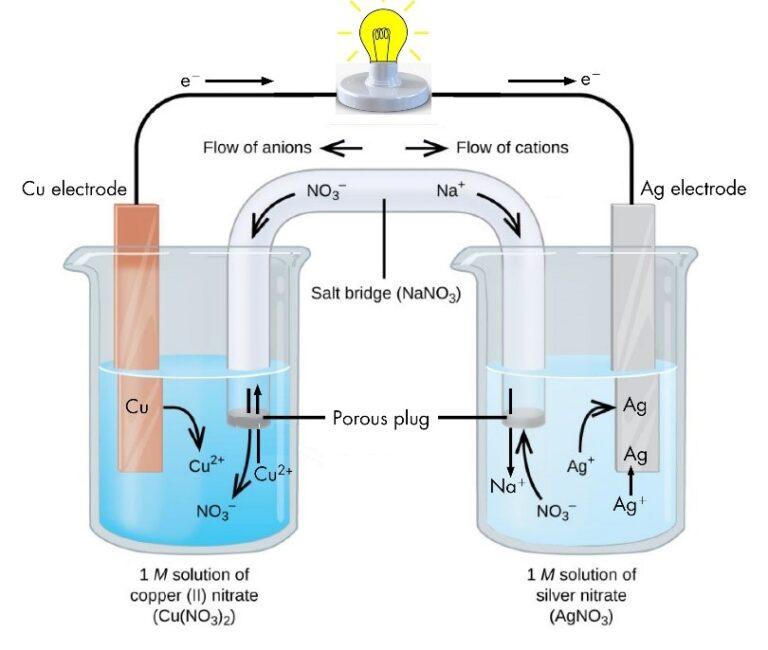
Electrochemistry Experiments Pdf . In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will work with both electrolytic. Chemical reactions involving the transfer of electrons from one reactant to another are. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. A) to determine the order of reactivity for four metals. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages.
from knowledgecycle.in
A) to determine the order of reactivity for four metals. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. You will work with both electrolytic. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Chemical reactions involving the transfer of electrons from one reactant to another are. Electrochemistry (2 period laboratory) the goals of the experiment:
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge
Electrochemistry Experiments Pdf In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. Chemical reactions involving the transfer of electrons from one reactant to another are. A) to determine the order of reactivity for four metals. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemistry (2 period laboratory) the goals of the experiment: You will work with both electrolytic. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages.
From sanet.st
Experimental Electrochemistry A Laboratory Textbook, 2nd Edition Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. You will work with both electrolytic. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment you will build various. Electrochemistry Experiments Pdf.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge Electrochemistry Experiments Pdf You will work with both electrolytic. A) to determine the order of reactivity for four metals. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment, you will have the chance to see. Electrochemistry Experiments Pdf.
From www.scribd.com
Question and Answer On Electrochemistry Redox Electrochemistry Electrochemistry Experiments Pdf Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will work with both electrolytic. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and. Electrochemistry Experiments Pdf.
From www.researchgate.net
(PDF) Electrochemistry for environmental remediation laboratory Electrochemistry Experiments Pdf Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. You will work with both electrolytic. Chemical reactions involving the transfer of electrons from. Electrochemistry Experiments Pdf.
From www.studocu.com
Electrochemical Experiments Electrochemistry Studocu Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. A) to determine the order of reactivity for four metals. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrochemistry (2 period laboratory) the goals of the experiment: Electrolysis is the decomposition. Electrochemistry Experiments Pdf.
From dokumen.tips
(PDF) 3. ELECTROCHEMISTRY GALVANIC CELLS 3. ELECTROCHEMISTRY Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. You will work with both electrolytic. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. A) to determine the order of reactivity for four metals. In this experiment we assemble an. Electrochemistry Experiments Pdf.
From worksheetzonehensley.z4.web.core.windows.net
Electrochemical Cell Pdf Notes Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrochemistry (2 period laboratory) the goals of. Electrochemistry Experiments Pdf.
From www.scribd.com
Electrochemistry Notes PDF Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. Chemical reactions involving the transfer of electrons from one reactant to another are. You will work with both electrolytic. Electrolysis is the decomposition of a molten. Electrochemistry Experiments Pdf.
From www.pinterest.com
Electrochemistry Electrochemistry, Chemistry experiments, Teaching Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment we assemble an electrolytic cell in which. Electrochemistry Experiments Pdf.
From www.scribd.com
Analysis of an Electrochemistry Experiment on Electrolysis and Voltaic Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. A) to determine the order of reactivity for four metals. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. In this experiment, you will have the chance to see how electron transfer processes are important in. Electrochemistry Experiments Pdf.
From saylordotorg.github.io
Electrochemistry Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. You will work with both electrolytic. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment, you will have the chance to see how electron transfer. Electrochemistry Experiments Pdf.
From studylib.net
Electrochemistry Electrochemistry Experiments Pdf In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrochemistry (2 period laboratory) the goals of the experiment: You will work with both electrolytic. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Chemical reactions involving the transfer of electrons from. Electrochemistry Experiments Pdf.
From www.researchgate.net
(PDF) Basics of Electrochemistry for Beginners Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will work with both electrolytic. A) to determine the order of reactivity for four metals. In this experiment we assemble an electrolytic cell in which one. Electrochemistry Experiments Pdf.
From www.scribd.com
Electrochemistry PDF Electrochemistry Experiments Pdf You will work with both electrolytic. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. A) to determine the order of reactivity for four metals. Electrochemistry (2 period laboratory) the. Electrochemistry Experiments Pdf.
From www.scribd.com
Electrochemistry of Immobilized Particles and Droplets Experiments with Electrochemistry Experiments Pdf You will work with both electrolytic. Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. A) to determine the order of reactivity. Electrochemistry Experiments Pdf.
From www.researchgate.net
(PDF) In situ electrochemical experiments Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrolysis is the decomposition of a molten. Electrochemistry Experiments Pdf.
From www.researchgate.net
(PDF) An Electrochemistry Experiment Hydrogen Evolution Reaction on Electrochemistry Experiments Pdf Electrochemistry (2 period laboratory) the goals of the experiment: A) to determine the order of reactivity for four metals. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrolysis is the decomposition. Electrochemistry Experiments Pdf.
From 2012books.lardbucket.org
Electrochemistry Electrochemistry Experiments Pdf Electrochemistry (2 period laboratory) the goals of the experiment: A) to determine the order of reactivity for four metals. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will. Electrochemistry Experiments Pdf.
From saylordotorg.github.io
Electrochemistry Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. You will work with both electrolytic. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemistry (2 period laboratory) the goals of the experiment: A) to determine the order of reactivity for four metals. In this. Electrochemistry Experiments Pdf.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. You will work with both electrolytic. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Chemical reactions involving the transfer of electrons from. Electrochemistry Experiments Pdf.
From www.yumpu.com
PDF/READ Electrochemistry (Oxford Chemistry Primers) full Electrochemistry Experiments Pdf Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Chemical reactions involving the transfer of electrons from one reactant to another are. A) to determine the order of reactivity for four metals. You will work with both electrolytic. In this experiment,. Electrochemistry Experiments Pdf.
From www.researchgate.net
1 The setup for the electrochemical experiments. Download Scientific Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. You will work with both electrolytic. Chemical reactions. Electrochemistry Experiments Pdf.
From www.studocu.com
Experiment2 report Experiment 2. Electrochemistry and Corrosion Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. A) to determine the order of reactivity for four metals. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Electrolysis is the decomposition of a molten or aqueous ionic. Electrochemistry Experiments Pdf.
From www.scribd.com
Experiment Electrochemistry PDF Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. Chemical reactions involving the transfer of electrons from one reactant to another are. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte). Electrochemistry Experiments Pdf.
From www.scribd.com
17 Electrochemistry with answers.pdf Electrochemistry Redox Electrochemistry Experiments Pdf A) to determine the order of reactivity for four metals. You will work with both electrolytic. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. Chemical. Electrochemistry Experiments Pdf.
From www.researchgate.net
Electrochemical experiment setup. Download Scientific Diagram Electrochemistry Experiments Pdf Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. In this experiment we assemble an electrolytic cell in which one electrode is a. Electrochemistry Experiments Pdf.
From studylib.net
Introduction to Electrochemistry Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode. Electrochemistry Experiments Pdf.
From www.studocu.com
Electrochemical cellsreport Investigating Electrochemical cells Aim Electrochemistry Experiments Pdf Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will work with both electrolytic. Electrochemistry (2 period laboratory) the goals of the experiment: A) to determine the order of reactivity for four metals. In this experiment, you will have the chance to see how electron transfer processes are. Electrochemistry Experiments Pdf.
From philschatz.com
Electrolysis · Chemistry Electrochemistry Experiments Pdf In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. You will work with both electrolytic. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric. Electrochemistry Experiments Pdf.
From saylordotorg.github.io
Electrochemistry Electrochemistry Experiments Pdf You will work with both electrolytic. Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. A) to determine the order of reactivity for four metals. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte). Electrochemistry Experiments Pdf.
From notesbooster.blogspot.com
Chapter 3 Electrochemistry Class 12 Handwritten Notes PDF download Electrochemistry Experiments Pdf In this experiment you will build various electrochemical cells using cu, zn, al, and fe and measure the voltages. Electrochemistry (2 period laboratory) the goals of the experiment: In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. In this experiment we assemble an electrolytic cell in which one electrode is a. Electrochemistry Experiments Pdf.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Electrochemistry Experiments Pdf In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. A) to determine the order of reactivity for four metals. Electrochemistry (2 period laboratory) the goals of the experiment: Chemical reactions involving the transfer of electrons. Electrochemistry Experiments Pdf.
From www.studocu.com
Electrochemistry Lab Chem 1B Electrochemistry Experiment 1 Standard Electrochemistry Experiments Pdf In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. You will work with both electrolytic. In this experiment we assemble an electrolytic cell in which one electrode is a strip of. Electrochemistry Experiments Pdf.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemistry Experiments Pdf You will work with both electrolytic. In this experiment, you will have the chance to see how electron transfer processes are important in chemistry. Electrochemistry (2 period laboratory) the goals of the experiment: Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment you will build various electrochemical cells using cu, zn, al, and. Electrochemistry Experiments Pdf.
From www.researchgate.net
Electrochemical experimental setup for studying the electrochemistry of Electrochemistry Experiments Pdf Chemical reactions involving the transfer of electrons from one reactant to another are. In this experiment we assemble an electrolytic cell in which one electrode is a strip of unknown metal, m, the other electrode is an inert metal (copper), and the electrolyte is an acidic solution. In this experiment you will build various electrochemical cells using cu, zn, al,. Electrochemistry Experiments Pdf.