Calorimeter Practice Problems . when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. in this set of practice questions, we will go over the main types of questions on calorimetry including the. calorimetry practice problems. One method of generating electricity is by burning coal to heat water, which produces steam that. How many joules of heat are required to raise the temperature of 1000. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and.
from www.slideserve.com
through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. How many joules of heat are required to raise the temperature of 1000. calorimetry practice problems. in this set of practice questions, we will go over the main types of questions on calorimetry including the. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. One method of generating electricity is by burning coal to heat water, which produces steam that. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? a calorimeter measures the amount of heat absorbed or released during a chemical or physical process.
PPT Calorimetry PowerPoint Presentation ID6655927
Calorimeter Practice Problems How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go over the main types of questions on calorimetry including the. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. One method of generating electricity is by burning coal to heat water, which produces steam that. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. How many joules of heat are required to raise the temperature of 1000.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Practice Problems How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. in this set of practice questions, we will go over the main types of questions on calorimetry including the. this online quiz is intended. Calorimeter Practice Problems.
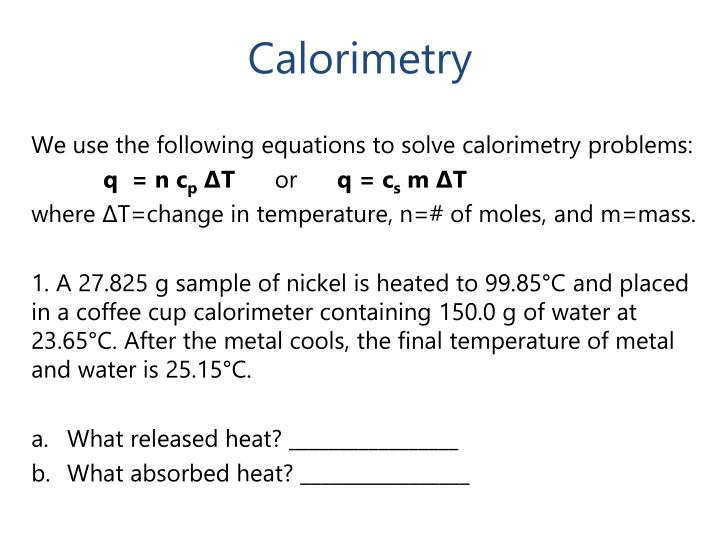
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Calorimeter Practice Problems in this set of practice questions, we will go over the main types of questions on calorimetry including the. calorimetry practice problems. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. One method of generating electricity is by burning coal to heat water, which produces steam that. How many joules. Calorimeter Practice Problems.
From giokbmnho.blob.core.windows.net
Calorimeter Constant Negative at Joanna Williams blog Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. calorimetry practice problems. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen. Calorimeter Practice Problems.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Practice Problems a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. How many joules of heat are required to raise the temperature of 1000. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. in this. Calorimeter Practice Problems.
From worksheetmediadwaum.z14.web.core.windows.net
Calorimetry Problems With Solutions Calorimeter Practice Problems in this set of practice questions, we will go over the main types of questions on calorimetry including the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems. One method of generating electricity is by burning coal to heat water, which produces steam that. when 1.0 g. Calorimeter Practice Problems.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. calorimetry practice problems. One method of generating electricity is by burning coal to heat water, which produces steam that. How much energy is needed to change the temperature of 50.0 g of. Calorimeter Practice Problems.
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Calorimeter Practice Problems in this set of practice questions, we will go over the main types of questions on calorimetry including the. calorimetry practice problems. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. a calorimeter measures the amount of heat absorbed. Calorimeter Practice Problems.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimeter Practice Problems How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems. in this set of practice questions, we will go over the main types of questions on calorimetry including the. One method of generating electricity is by burning coal to heat water, which produces steam that. How many joules of. Calorimeter Practice Problems.
From studylib.net
Heat Practice Problems Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. How many joules of heat are required to raise the temperature of 1000. How much energy is needed to change the temperature of 50.0 g of water. Calorimeter Practice Problems.
From www.studocu.com
Calorimetry Worksheet H = ms T H = (5,650 grams H 2 O) (4 J/g °C)(55 Calorimeter Practice Problems a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. in this set of practice questions, we will go over the main types of questions on calorimetry including the. How many joules of heat. Calorimeter Practice Problems.
From studylib.net
Thermochemistry Practice Problems Calorimeter Practice Problems How many joules of heat are required to raise the temperature of 1000. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. this online quiz is intended to give you extra practice in. Calorimeter Practice Problems.
From exobwfkeu.blob.core.windows.net
Calorimeter Problems And Answers at Christopher Dominguez blog Calorimeter Practice Problems this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. How many joules of heat are required to raise the temperature. Calorimeter Practice Problems.
From www.studocu.com
Heat Capacity and Specific Heat Calculations Practice A 1 g sample Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. calorimetry practice problems. in this set of practice questions, we will go over the main types of questions on calorimetry including the. How many joules of heat are required to raise the temperature of 1000. a calorimeter measures the amount of. Calorimeter Practice Problems.
From www.studocu.com
Calorimetry Practice Problems a. How much energy is needed to raise Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. calorimetry practice problems. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. through these exercises, you will have the opportunity to apply the theoretical. Calorimeter Practice Problems.
From exobwfkeu.blob.core.windows.net
Calorimeter Problems And Answers at Christopher Dominguez blog Calorimeter Practice Problems this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. One method of generating electricity is by burning coal to heat water, which produces steam that. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How many joules of heat are. Calorimeter Practice Problems.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. this online quiz is intended to give you extra practice in calorimetry problems (q =. Calorimeter Practice Problems.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. How many joules of heat are required to raise. Calorimeter Practice Problems.
From www.chegg.com
Solved CALORIMETER PRACTICE PROBLEMS 1. A 25 gram sample of Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?. Calorimeter Practice Problems.
From www.chegg.com
Solved A coffee cup calorimeter contains 151.41 g of water Calorimeter Practice Problems in this set of practice questions, we will go over the main types of questions on calorimetry including the. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. How many joules of heat are required to raise the temperature of 1000. when 1.0 g of. Calorimeter Practice Problems.
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. One method of generating electricity is by burning coal to heat water, which produces steam that. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? when 1.0 g of fructose, c 6 h 12. Calorimeter Practice Problems.
From www.studocu.com
Bomb Calorimeter Problems Bomb Calorimeter Practice Problems Name Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. in this set of practice questions, we will go over the main types of questions on calorimetry including the. One method of generating electricity is by burning coal to heat water, which produces steam that. this online quiz is intended. Calorimeter Practice Problems.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. calorimetry practice. Calorimeter Practice Problems.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. One method of generating electricity is by burning coal to heat water, which produces steam that. in this set of practice questions, we will. Calorimeter Practice Problems.
From www.slideserve.com
PPT Chemical Reactions / Thermochemistry / Gases PowerPoint Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. calorimetry practice problems. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. One method of generating electricity is by burning coal to heat. Calorimeter Practice Problems.
From www.numerade.com
SOLVED Calorimetry Problem Set For each problem below, write the Calorimeter Practice Problems a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. calorimetry practice problems. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go over the main types of questions on calorimetry including the. How many joules. Calorimeter Practice Problems.
From sweetnowsw.blogspot.com
Calorimetry Problems Worksheet Specific Heat And Calorimetry Packet Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?. Calorimeter Practice Problems.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimeter Practice Problems through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. One method of generating electricity is by burning coal to heat water, which produces steam that. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. when 1.0 g of fructose, c 6 h. Calorimeter Practice Problems.
From www.youtube.com
Chapter 09 17 PROBLEM Coffee Cup Calorimeter YouTube Calorimeter Practice Problems a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. in this set of practice questions, we will go over the main types of questions on calorimetry including the. How many joules of heat are required to raise the temperature of 1000. One method of generating electricity is by burning coal to. Calorimeter Practice Problems.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimeter Practice Problems How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. in this set of practice questions, we will go over the main types of questions on calorimetry including the. calorimetry practice problems. One method of. Calorimeter Practice Problems.
From deboramilke.blogspot.com
Bomb Calorimetry Problems Debora Milke Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. calorimetry practice problems. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and.. Calorimeter Practice Problems.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimeter Practice Problems calorimetry practice problems. How many joules of heat are required to raise the temperature of 1000. a calorimeter measures the amount of heat absorbed or released during a chemical or physical process. when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb. Calorimeter Practice Problems.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. How many joules of heat are required to raise the temperature of 1000. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for. Calorimeter Practice Problems.
From studylib.net
Calorimetry Worksheet Calorimeter Practice Problems when 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter,. How many joules of heat are required to raise the temperature of 1000. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. a. Calorimeter Practice Problems.
From studylib.net
CALORIMETER PRACTICE PROBLEMS 1 1. Find the specific Calorimeter Practice Problems this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. calorimetry practice problems. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? One method of generating electricity is by burning coal to heat water, which produces steam that. through. Calorimeter Practice Problems.
From studylib.net
10.3 Calorimetry Practice Problems Name________________________________ H Calorimeter Practice Problems One method of generating electricity is by burning coal to heat water, which produces steam that. this online quiz is intended to give you extra practice in calorimetry problems (q = c⋅m⋅δt) with options for different units. through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and. in this set. Calorimeter Practice Problems.