Bromide Ion Test . chlorine, bromine and iodine are halogens. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. A cream precipitate indicates the presence of the bromide ion. bromide and iodide ions give similarly coloured precipitates. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. Halide ions in solutions are detected. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. a white precipitate indicates the presence of the chloride ion. this experiment involves identifying the cations and anions in various salt solutions. It’s helpful for students to know. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. testing for halide ions.
from www.shutterstock.com
this experiment involves identifying the cations and anions in various salt solutions. It’s helpful for students to know. Halide ions in solutions are detected. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. a white precipitate indicates the presence of the chloride ion. bromide and iodide ions give similarly coloured precipitates. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. testing for halide ions.
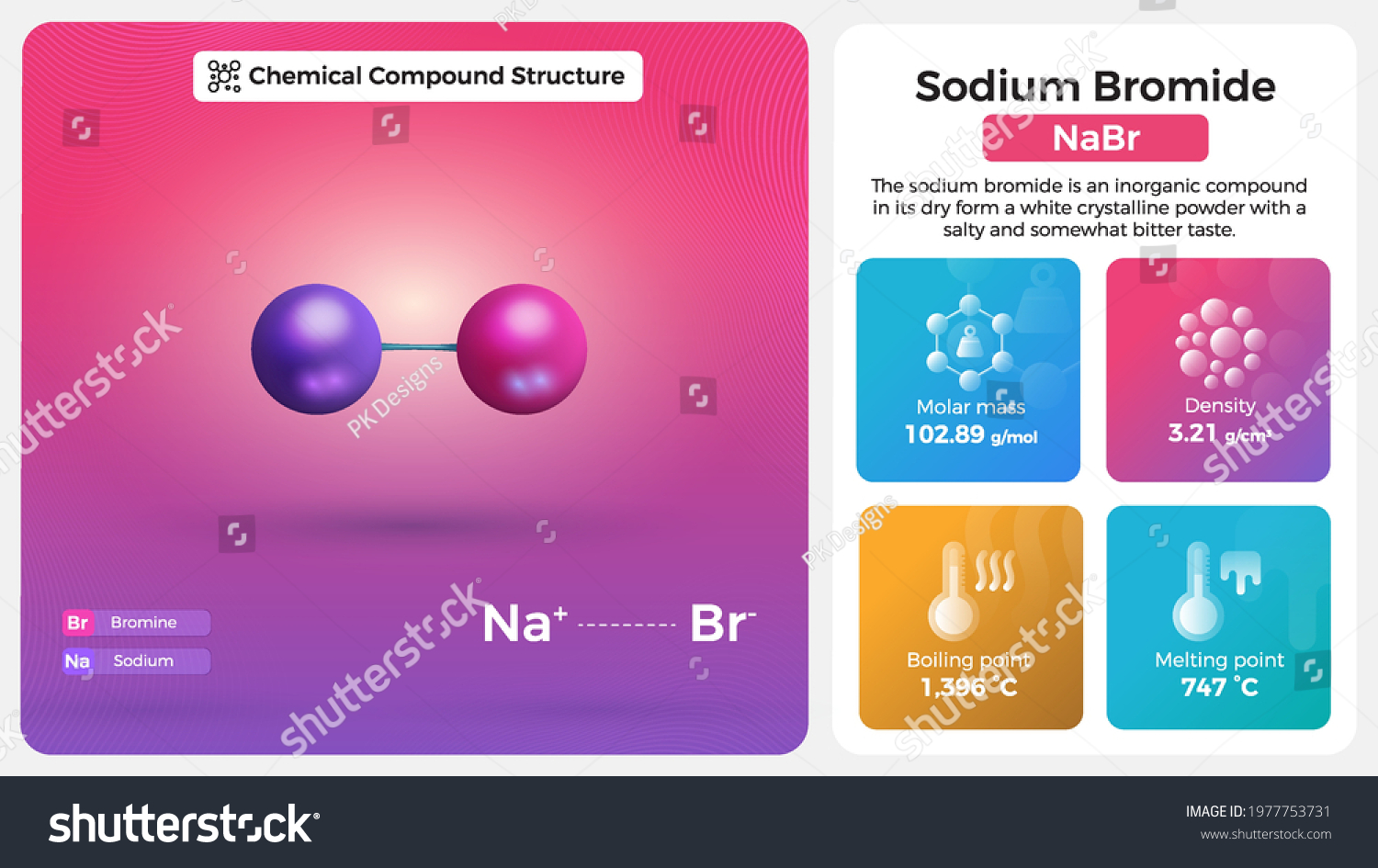
Sodium Bromide Properties Chemical Compound Structure Stock Vector
Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. It’s helpful for students to know. this experiment involves identifying the cations and anions in various salt solutions. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. A cream precipitate indicates the presence of the bromide ion. chlorine, bromine and iodine are halogens. bromide and iodide ions give similarly coloured precipitates. Halide ions in solutions are detected. a white precipitate indicates the presence of the chloride ion. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. testing for halide ions.
From slideplayer.com
15/09/ /09/2019 Chemical Analysis AQA 2016 Chemistry topic ppt download Bromide Ion Test this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. bromide and iodide ions give similarly coloured precipitates. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. A cream precipitate indicates the presence of the bromide. Bromide Ion Test.
From www.youtube.com
Test for Bromide ion Manganese dioxide test souravsir Bromide Ion Test A cream precipitate indicates the presence of the bromide ion. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. a white precipitate indicates the presence of the chloride. Bromide Ion Test.
From www.youtube.com
TEST FOR HALIDE IONS (Chloride, Bromide and Iodide ions) 3D animation Bromide Ion Test this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. A cream precipitate indicates the presence of the bromide ion. chlorine, bromine and iodine are halogens. testing for halide ions. Halide ions in solutions are detected. bromide and iodide ions give similarly coloured precipitates. a white precipitate. Bromide Ion Test.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. bromide and iodide ions give similarly coloured precipitates. chlorine, bromine and iodine are halogens. a white precipitate indicates the presence of the chloride ion. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. this page. Bromide Ion Test.
From www.science-revision.co.uk
Testing for anions Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. It’s helpful for students to know. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. bromide and iodide ions give similarly coloured precipitates. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. . Bromide Ion Test.
From www.savemyexams.com
Testing for Halide Ions AQA A Level Chemistry Revision Notes 2017 Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. bromide and iodide ions give similarly coloured precipitates. chlorine, bromine and iodine are halogens.. Bromide Ion Test.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Ion Test It’s helpful for students to know. testing for halide ions. this experiment involves identifying the cations and anions in various salt solutions. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. learn about. Bromide Ion Test.
From www.sigmaaldrich.co.th
THIAZOLYL BLUE TETRAZOLIU M BROMIDE, 98 Merck Life Sciences Thailand Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. It’s helpful for students to know. chlorine, bromine and. Bromide Ion Test.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Bromide Ion Test chlorine, bromine and iodine are halogens. this experiment involves identifying the cations and anions in various salt solutions. It’s helpful for students to know. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. bromide and iodide ions give similarly coloured precipitates. a white precipitate indicates the. Bromide Ion Test.
From www.flinnsci.ca
Sodium Bromide, Reagent, 100 g Flinn Scientific Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. a white precipitate indicates the presence of the chloride ion. testing for halide ions. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. chlorine, bromine and iodine are halogens. It’s helpful for students to know. Halide. Bromide Ion Test.
From askfilo.com
Practical No. 1 Aim To identify the chloride, bromide and iodide ions f.. Bromide Ion Test chlorine, bromine and iodine are halogens. bromide and iodide ions give similarly coloured precipitates. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. testing for halide ions. a white precipitate indicates the presence of the chloride ion. This page describes and explains the tests for halide ions (fluoride,. Bromide Ion Test.
From www.studocu.com
Ion Bromide 6 Bromide Ion Test in KBr Test This aim For identify Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. A cream precipitate indicates the presence of the bromide ion. It’s helpful for students to know.. Bromide Ion Test.
From www.youtube.com
Sodium bromide YouTube Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. chlorine, bromine and iodine are halogens. a white precipitate indicates the presence of the chloride ion. bromide and iodide ions give similarly coloured precipitates.. Bromide Ion Test.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Ion Test bromide and iodide ions give similarly coloured precipitates. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. a white precipitate indicates the presence of the chloride ion. this experiment involves identifying the cations and anions in various salt solutions. This page describes and. Bromide Ion Test.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Bromide Ion Test It’s helpful for students to know. this experiment involves identifying the cations and anions in various salt solutions. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. A cream precipitate indicates the presence of the bromide ion. Halide ions in solutions are detected. bromide and iodide ions give. Bromide Ion Test.
From www.youtube.com
Test for Bromide(Br) ion Acid Radical Analysis chemistrypractical Bromide Ion Test a white precipitate indicates the presence of the chloride ion. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. bromide and iodide ions give similarly coloured precipitates. Halide ions in solutions are detected. this experiment involves identifying the cations and anions in various. Bromide Ion Test.
From www.slideshare.net
Oxidation & reduction Bromide Ion Test a white precipitate indicates the presence of the chloride ion. A cream precipitate indicates the presence of the bromide ion. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. testing for halide ions. bromide and iodide ions give similarly coloured precipitates. It’s helpful. Bromide Ion Test.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Ion Test chlorine, bromine and iodine are halogens. bromide and iodide ions give similarly coloured precipitates. A cream precipitate indicates the presence of the bromide ion. testing for halide ions. It’s helpful for students to know. a white precipitate indicates the presence of the chloride ion. learn about and revise tests for ions with this bbc bitesize. Bromide Ion Test.
From www.ntsensors.com
Bromide IonSelective Electrode + probe NT Sensors Bromide Ion Test chlorine, bromine and iodine are halogens. A cream precipitate indicates the presence of the bromide ion. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. Halide ions in solutions are detected. testing for halide ions. It’s helpful for students to know. a white. Bromide Ion Test.
From www.slideshare.net
Lesson 2 Testing And Identifying Halide Ions Bromide Ion Test revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. chlorine, bromine and iodine are halogens. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. It’s helpful for students to know. testing for halide ions. Halide ions. Bromide Ion Test.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Bromide Ion Test Halide ions in solutions are detected. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. testing for halide ions. this experiment involves identifying the cations and anions. Bromide Ion Test.
From www.bigstockphoto.com
Test Bromides Iodides Image & Photo (Free Trial) Bigstock Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. this experiment involves identifying the cations and anions in various salt solutions. chlorine, bromine and iodine are halogens. bromide and. Bromide Ion Test.
From www.reddit.com
How does the negatively charged bromide ion from the heterolytic Bromide Ion Test this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. Halide ions in solutions are detected. bromide and iodide ions give similarly coloured precipitates. It’s helpful for students to know. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. This page describes. Bromide Ion Test.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Ion Test this experiment involves identifying the cations and anions in various salt solutions. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. chlorine, bromine and iodine are halogens. A cream precipitate indicates the presence of the bromide ion. It’s helpful for students to know. Halide ions in solutions are. Bromide Ion Test.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Ion Test a white precipitate indicates the presence of the chloride ion. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. bromide and iodide ions give similarly coloured precipitates. testing for halide ions. It’s helpful for students to know. This page describes and explains the tests for halide ions. Bromide Ion Test.
From www.youtube.com
Bromide Identification Test Anion Salt Analysis Detection of Bromide Ion Test chlorine, bromine and iodine are halogens. It’s helpful for students to know. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. testing for halide ions. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. bromide. Bromide Ion Test.
From www.youtube.com
Testing For Chlorides, Bromides & Iodides Chemical Tests Chemistry Bromide Ion Test It’s helpful for students to know. A cream precipitate indicates the presence of the bromide ion. a white precipitate indicates the presence of the chloride ion. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver. Bromide Ion Test.
From www.youtube.com
Layer Test for Bromide using Sodium Bromide YouTube Bromide Ion Test learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. chlorine, bromine and iodine are halogens. a white precipitate indicates the presence of the chloride ion. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. testing for halide ions. Halide ions in solutions are detected.. Bromide Ion Test.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Bromide Ion Test testing for halide ions. A cream precipitate indicates the presence of the bromide ion. It’s helpful for students to know. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. bromide and iodide ions give similarly coloured precipitates. Halide ions in solutions are detected. this experiment involves identifying the cations. Bromide Ion Test.
From sielc.com
Vinyl bromide SIELC Technologies Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. testing for halide ions. A cream precipitate indicates the. Bromide Ion Test.
From pubs.acs.org
The Multiple Role of Bromide Ion in PPCPs Degradation under UV/Chlorine Bromide Ion Test It’s helpful for students to know. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. chlorine, bromine and iodine are halogens. bromide and iodide ions give similarly coloured precipitates. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide. A cream. Bromide Ion Test.
From www.shalom-education.com
Testing for Halide ions GCSE Chemistry Revision Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. Halide ions in solutions are detected. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. learn about and revise tests for ions with this bbc bitesize gcse chemistry (edexcel) study guide.. Bromide Ion Test.
From www.youtube.com
Testing for chlorides, bromides and iodides Chemistry FuseSchool Bromide Ion Test This page describes and explains the tests for halide ions (fluoride, chloride, bromide and. a white precipitate indicates the presence of the chloride ion. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. It’s helpful for students to know. Halide ions in solutions are detected. learn about and. Bromide Ion Test.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Ion Test a white precipitate indicates the presence of the chloride ion. chlorine, bromine and iodine are halogens. It’s helpful for students to know. revision notes on 2.3.3 testing for halide ions for the aqa a level chemistry syllabus, written by the chemistry experts at save. this page discusses the tests for halide ions (fluoride, chloride, bromide and. Bromide Ion Test.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Ion Test a white precipitate indicates the presence of the chloride ion. bromide and iodide ions give similarly coloured precipitates. It’s helpful for students to know. this page discusses the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate and ammonia. this experiment involves identifying the cations and anions in various salt solutions. Halide ions. Bromide Ion Test.