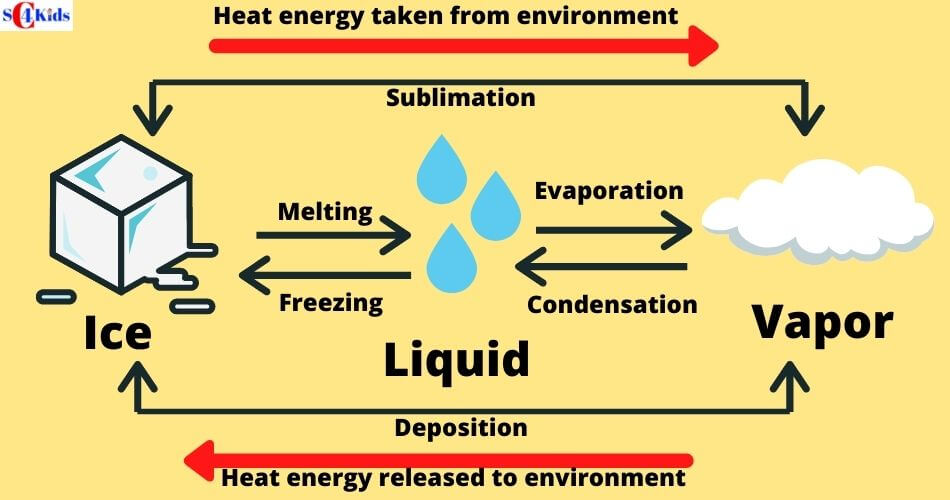
Solid To Liquid Heat Absorbed Or Released . The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident.
from smartclass4kids.com
when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. The energy per unit mass. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus).
Changing States of Matter Solid, Liquid,Gas, Phase Change
Solid To Liquid Heat Absorbed Or Released The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The conversion of a solid to a liquid is called fusion (or melting). when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. The energy per unit mass.
From slideplayer.com
1st Law of Thermodynamics Heat Transfer ppt download Solid To Liquid Heat Absorbed Or Released The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). the number of bonds is proportional to the number of molecules and thus to the mass. Solid To Liquid Heat Absorbed Or Released.
From www.chegg.com
Solved Sublimation Heat absorbed (80 cal) SOLID LIQUID GAS Solid To Liquid Heat Absorbed Or Released the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the latent heat of fusion is the amount of heat needed to cause a. Solid To Liquid Heat Absorbed Or Released.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The conversion of a solid to a liquid is called fusion (or melting). . Solid To Liquid Heat Absorbed Or Released.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid To Liquid Heat Absorbed Or Released The energy per unit mass. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is. Solid To Liquid Heat Absorbed Or Released.
From www.slideserve.com
PPT The States of Matter PowerPoint Presentation, free download ID Solid To Liquid Heat Absorbed Or Released the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The energy per unit mass. the latent heat of fusion is the amount of heat needed to cause a. Solid To Liquid Heat Absorbed Or Released.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The conversion of a solid to a liquid is called fusion (or melting).. Solid To Liquid Heat Absorbed Or Released.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid To Liquid Heat Absorbed Or Released the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The conversion of a solid to a liquid is called fusion (or melting). The energy per unit mass. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the. Solid To Liquid Heat Absorbed Or Released.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Solid To Liquid Heat Absorbed Or Released The energy per unit mass. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. the number of bonds is proportional to the number of. Solid To Liquid Heat Absorbed Or Released.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Heat Absorbed Or Released the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. The energy required to melt 1 mol of a substance is its enthalpy. Solid To Liquid Heat Absorbed Or Released.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint Solid To Liquid Heat Absorbed Or Released The energy per unit mass. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is. Solid To Liquid Heat Absorbed Or Released.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the. Solid To Liquid Heat Absorbed Or Released.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Solid To Liquid Heat Absorbed Or Released when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. the number of bonds is proportional to the number of molecules and thus to the. Solid To Liquid Heat Absorbed Or Released.
From opentextbc.ca
Phase Changes Basic HVAC Solid To Liquid Heat Absorbed Or Released The conversion of a solid to a liquid is called fusion (or melting). during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The energy per unit mass.. Solid To Liquid Heat Absorbed Or Released.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The energy per unit mass. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the number of bonds is proportional to the number of molecules and thus to the mass of the sample. during phase changes, heat absorbed or released is given by \(q =. Solid To Liquid Heat Absorbed Or Released.
From www.processtechacademy.com
Proc Tech & Oper Acad Causes of Higher Process Temps Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point. Solid To Liquid Heat Absorbed Or Released.
From exovqqtpg.blob.core.windows.net
What Should The Heat Be Set At at Beth Gray blog Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The energy per unit mass. the number of bonds is proportional to the number of molecules. Solid To Liquid Heat Absorbed Or Released.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. when heat energy is supplied to a solid (like ice) at a steady. Solid To Liquid Heat Absorbed Or Released.
From www.vrogue.co
Heat Absorbed Formulas How To Calculate It And Solved vrogue.co Solid To Liquid Heat Absorbed Or Released The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the number of bonds. Solid To Liquid Heat Absorbed Or Released.
From mungfali.com
Solid Liquid Gas Anchor Chart Solid To Liquid Heat Absorbed Or Released The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the number of bonds is proportional to the number of molecules and thus to the mass. Solid To Liquid Heat Absorbed Or Released.
From www.sciencelearn.org.nz
Heat and change of state — Science Learning Hub Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. The energy per unit mass. the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The conversion of a solid to a liquid is. Solid To Liquid Heat Absorbed Or Released.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). The energy per unit mass. the latent heat of fusion is the amount of heat needed to cause a phase. Solid To Liquid Heat Absorbed Or Released.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). . Solid To Liquid Heat Absorbed Or Released.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Solid To Liquid Heat Absorbed Or Released The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become. Solid To Liquid Heat Absorbed Or Released.
From www.coursehero.com
[Solved] Calculate the amount of heat absorbed or released by the Solid To Liquid Heat Absorbed Or Released the number of bonds is proportional to the number of molecules and thus to the mass of the sample. The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The energy per unit mass.. Solid To Liquid Heat Absorbed Or Released.
From brainly.in
In the diagram below, label all of the arrows as either Heat Released Solid To Liquid Heat Absorbed Or Released The energy per unit mass. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. The energy required to melt 1 mol of a substance is. Solid To Liquid Heat Absorbed Or Released.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Heat Absorbed Or Released when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. The energy per unit mass. The energy required to melt 1 mol of a substance is. Solid To Liquid Heat Absorbed Or Released.
From www.slideserve.com
PPT CHAPTER 7 HEAT PowerPoint Presentation, free download ID2090526 Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). the number of bonds is proportional to the number of molecules and thus to the mass of the sample. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. The. Solid To Liquid Heat Absorbed Or Released.
From sciencenotes.org
Endothermic Reactions Definition and Examples Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first. Solid To Liquid Heat Absorbed Or Released.
From www.pinterest.com
Latent Heat the heat energy that must be absorbed when a substance Solid To Liquid Heat Absorbed Or Released the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs. Solid To Liquid Heat Absorbed Or Released.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint Solid To Liquid Heat Absorbed Or Released The conversion of a solid to a liquid is called fusion (or melting). the number of bonds is proportional to the number of molecules and thus to the mass of the sample. when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature. Solid To Liquid Heat Absorbed Or Released.
From slideplayer.com
Heat &Heating Curves The change in the internal energy of a substance Solid To Liquid Heat Absorbed Or Released the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The energy per unit mass. during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. the number of bonds is proportional to the number of molecules and. Solid To Liquid Heat Absorbed Or Released.
From www.tec-science.com
Specific latent heat of condensation tecscience Solid To Liquid Heat Absorbed Or Released the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. The conversion of a solid to a liquid is called fusion (or melting). The energy per unit mass. The energy per unit mass. when heat energy is supplied to a solid (like ice) at a steady rate by. Solid To Liquid Heat Absorbed Or Released.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Solid To Liquid Heat Absorbed Or Released The conversion of a solid to a liquid is called fusion (or melting). when heat energy is supplied to a solid (like ice) at a steady rate by means of an electrical heating coil, we find that the temperature climbs steadily until the melting point is reached and the first signs of liquid formation become evident. The energy per. Solid To Liquid Heat Absorbed Or Released.
From slideplayer.com
Heat and Temperature Almost every chemical reaction is by a Solid To Liquid Heat Absorbed Or Released The energy per unit mass. The conversion of a solid to a liquid is called fusion (or melting). the number of bonds is proportional to the number of molecules and thus to the mass of the sample. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. . Solid To Liquid Heat Absorbed Or Released.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3746915 Solid To Liquid Heat Absorbed Or Released during phase changes, heat absorbed or released is given by \(q = ml,\) where \(l\) is the latent heat coefficient. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. when heat energy is supplied to a solid (like ice) at a steady rate by means of. Solid To Liquid Heat Absorbed Or Released.