Titrations Chemical Indicators . In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Every titration uses the same players: The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know. there are two basic types of acid base titrations, indicator and potentiometric. how titrations work and what you need. A graph is shown below where ph against the volume of base added is considered.
from www.studocu.com
A graph is shown below where ph against the volume of base added is considered. how titrations work and what you need. there are two basic types of acid base titrations, indicator and potentiometric. Every titration uses the same players: The analyte (titrand) is the solution with an unknown molarity. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions.
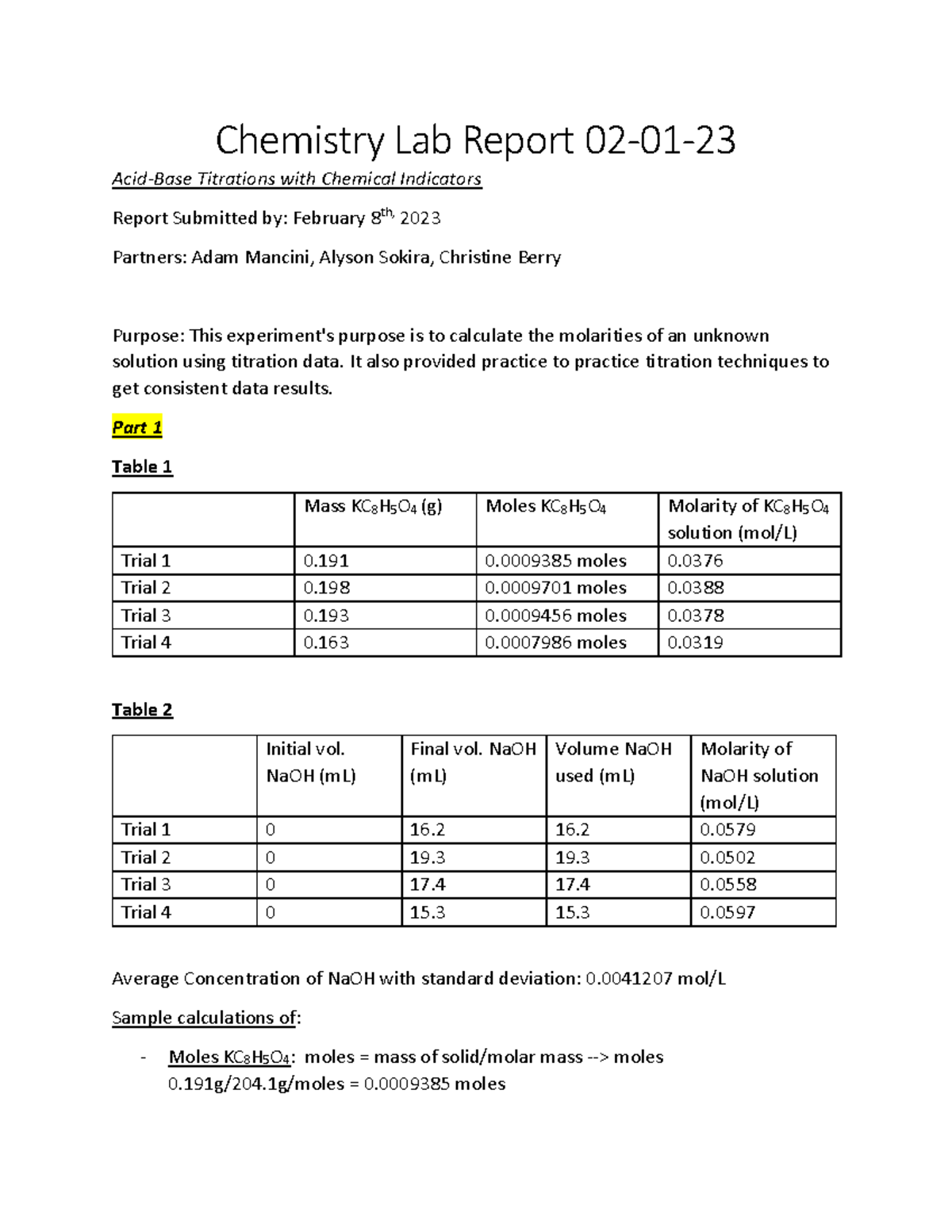
AcidBase Titrations with Chemical Indicators Lab Report 020123
Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. Every titration uses the same players: how titrations work and what you need. A graph is shown below where ph against the volume of base added is considered. The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In an indicator based titration you add another chemical that changes color at the ph. Titrations Chemical Indicators.
From dxofrcamx.blob.core.windows.net
What Is The Meaning Of Indicator In Chemistry at Laura Nelson blog Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. how titrations work and what you need. This page assumes that you know. A graph is shown below where ph against the volume of base added is considered. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of. Titrations Chemical Indicators.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titrations Chemical Indicators there are two basic types of acid base titrations, indicator and potentiometric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the same players: In an indicator based titration you. Titrations Chemical Indicators.
From www.hbarsci.com
Using Titrations, Chemical Analysis Kit Up to 10 Groups — hBARSCI Titrations Chemical Indicators there are two basic types of acid base titrations, indicator and potentiometric. A graph is shown below where ph against the volume of base added is considered. The analyte (titrand) is the solution with an unknown molarity. Every titration uses the same players: how titrations work and what you need. This page assumes that you know. The reagent. Titrations Chemical Indicators.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titrations Chemical Indicators This page assumes that you know. The analyte (titrand) is the solution with an unknown molarity. A graph is shown below where ph against the volume of base added is considered. Every titration uses the same players: The reagent (titrant) is the solution with a known molarity that will react with the analyte. In an indicator based titration you add. Titrations Chemical Indicators.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titrations Chemical Indicators how titrations work and what you need. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The graph shows the results obtained using. Titrations Chemical Indicators.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Titrations Chemical Indicators.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titrations Chemical Indicators This page assumes that you know. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The reagent (titrant) is the solution with a known molarity that will react with the analyte. A graph is shown below where ph against the. Titrations Chemical Indicators.
From studylib.net
AcidBase Titrations Indicators Analytical Chemistry Titrations Chemical Indicators The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. how titrations work and what you need. there are two basic types of acid base titrations, indicator and potentiometric. In an indicator based. Titrations Chemical Indicators.
From mungfali.com
Acid Base Titration Indicator Titrations Chemical Indicators In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid). Titrations Chemical Indicators.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titrations Chemical Indicators Every titration uses the same players: The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. how titrations work and what you need. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add. Titrations Chemical Indicators.
From www.hbarsci.com
Using Titrations, Chemical Analysis Kit Up to 10 Groups — hBARSCI Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. how titrations work and what you need. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. This page assumes that you know. The reagent (titrant) is the solution with. Titrations Chemical Indicators.
From www.scribd.com
AcidBase Titrations Indicators PDF Acid Chemistry Titrations Chemical Indicators Every titration uses the same players: In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two basic types of acid base titrations, indicator and potentiometric. how titrations work and what you need. The graph shows the. Titrations Chemical Indicators.
From www.microlit.com
An Advanced Guide to Titration Microlit Titrations Chemical Indicators This page assumes that you know. Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The analyte (titrand) is the solution. Titrations Chemical Indicators.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titrations Chemical Indicators there are two basic types of acid base titrations, indicator and potentiometric. This page assumes that you know. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators (methyl red and. Titrations Chemical Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titrations Chemical Indicators how titrations work and what you need. The analyte (titrand) is the solution with an unknown molarity. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In an indicator based titration you add. Titrations Chemical Indicators.
From dxozxirwd.blob.core.windows.net
Chemical Indicator In A Titration at Nicholas Terrell blog Titrations Chemical Indicators The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know. there are two basic types of acid base titrations, indicator and potentiometric. Every titration uses the same players: In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base. Titrations Chemical Indicators.
From www.hbarsci.com
Using Titrations, Chemical Analysis Kit Up to 10 Groups — hBARSCI Titrations Chemical Indicators how titrations work and what you need. Every titration uses the same players: A graph is shown below where ph against the volume of base added is considered. The reagent (titrant) is the solution with a known molarity that will react with the analyte. there are two basic types of acid base titrations, indicator and potentiometric. In an. Titrations Chemical Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titrations Chemical Indicators there are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. how titrations work and what you need. The graph shows the results obtained using two indicators (methyl. Titrations Chemical Indicators.
From saylordotorg.github.io
AcidBase Titrations Titrations Chemical Indicators This page assumes that you know. A graph is shown below where ph against the volume of base added is considered. Every titration uses the same players: In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. there are two. Titrations Chemical Indicators.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titrations Chemical Indicators how titrations work and what you need. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Titrations Chemical Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titrations Chemical Indicators A graph is shown below where ph against the volume of base added is considered. there are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. Every titration uses the same players: how titrations work and what you need. The analyte. Titrations Chemical Indicators.
From mungfali.com
Acid Base Titration Indicator Titrations Chemical Indicators This page assumes that you know. Every titration uses the same players: The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators. Titrations Chemical Indicators.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titrations Chemical Indicators how titrations work and what you need. A graph is shown below where ph against the volume of base added is considered. This page assumes that you know. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid). Titrations Chemical Indicators.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Titrations Chemical Indicators This page assumes that you know. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In an indicator. Titrations Chemical Indicators.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titrations Chemical Indicators The reagent (titrant) is the solution with a known molarity that will react with the analyte. Every titration uses the same players: The analyte (titrand) is the solution with an unknown molarity. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak. Titrations Chemical Indicators.
From www.studocu.com
AcidBase Titrations with Chemical Indicators Lab Report 020123 Titrations Chemical Indicators The reagent (titrant) is the solution with a known molarity that will react with the analyte. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know. there are. Titrations Chemical Indicators.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Titrations Chemical Indicators Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. how titrations work and what you need. A graph is shown below where ph against the volume of base added is considered. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another. Titrations Chemical Indicators.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titrations Chemical Indicators In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid). Titrations Chemical Indicators.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titrations Chemical Indicators there are two basic types of acid base titrations, indicator and potentiometric. Every titration uses the same players: In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. how titrations work and what you need. The reagent (titrant) is. Titrations Chemical Indicators.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Titrations Chemical Indicators A graph is shown below where ph against the volume of base added is considered. The analyte (titrand) is the solution with an unknown molarity. Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration. Titrations Chemical Indicators.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Titrations Chemical Indicators Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. A graph is shown below where ph against the volume of base added is considered.. Titrations Chemical Indicators.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titrations Chemical Indicators A graph is shown below where ph against the volume of base added is considered. how titrations work and what you need. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The graph shows the results obtained using two. Titrations Chemical Indicators.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Titrations Chemical Indicators how titrations work and what you need. there are two basic types of acid base titrations, indicator and potentiometric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the. Titrations Chemical Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titrations Chemical Indicators The reagent (titrant) is the solution with a known molarity that will react with the analyte. Every titration uses the same players: there are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. how titrations work and what you need. A graph is shown below where ph. Titrations Chemical Indicators.