Why Does Temperature Rise With Pressure . In reality, most compression take place by reducing volume. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. But if the same data is used and the graph extended to the left, at what. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Learn more about this in this article. That is, the atmospheric gas exerts less. We can understand this using an idea called the. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased;
from garciaspeausell.blogspot.com
We can understand this using an idea called the. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. That is, the atmospheric gas exerts less. Learn more about this in this article. But if the same data is used and the graph extended to the left, at what. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. The energy added as work during the compression of a gas leads to an increase in pressure and temperature.
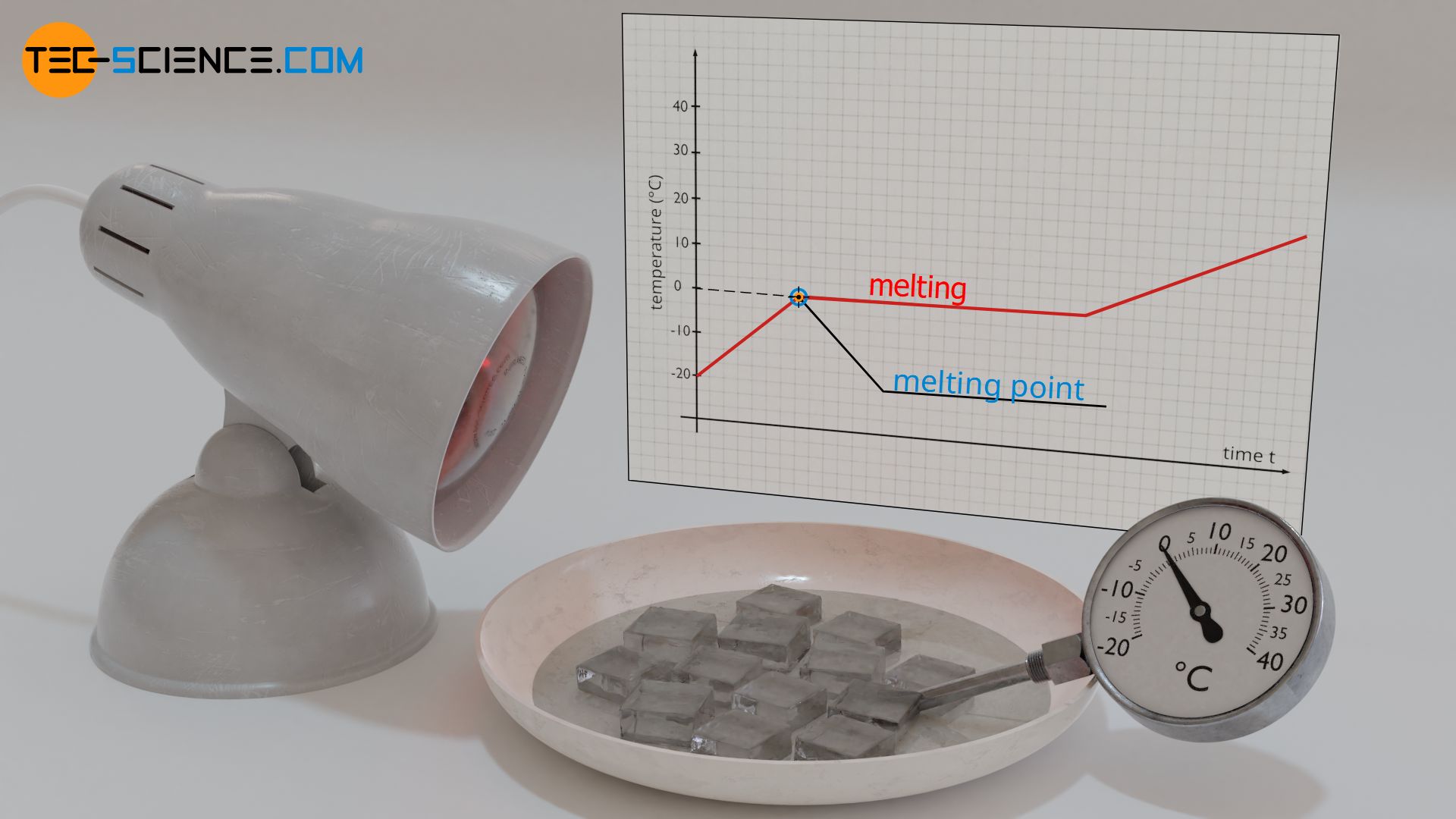
Why Did the Temperature Rise to a Constant Temperature Instead of
Why Does Temperature Rise With Pressure In reality, most compression take place by reducing volume. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Learn more about this in this article. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? The energy added as work during the compression of a gas leads to an increase in pressure and temperature. We can understand this using an idea called the. That is, the atmospheric gas exerts less. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; In reality, most compression take place by reducing volume. But if the same data is used and the graph extended to the left, at what. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube Why Does Temperature Rise With Pressure We can understand this using an idea called the. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. But if the same data is used and the graph extended to the left, at what. In reality, most compression take place by reducing. Why Does Temperature Rise With Pressure.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Does Temperature Rise With Pressure This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. That is, the atmospheric gas exerts less. If you had a way to increase pressure. Why Does Temperature Rise With Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Temperature Rise With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. We can understand this using an idea called the. But if the same data is used and the graph extended to the left, at what. Increasing temperature increases the average kinetic energy (ke) and the average velocity of. Why Does Temperature Rise With Pressure.
From www.researchgate.net
Why there is Wait Gain instead of Wait Loss in PTGA analysis with Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. In reality, most compression take place by reducing volume. Learn more about this in this article. That is, the atmospheric gas exerts less. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? Weather balloons. Why Does Temperature Rise With Pressure.
From relationrise.com
What Is The Relationship Between Pressure And Temperature Why Does Temperature Rise With Pressure Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? We can understand this using an idea called the. Weather balloons get larger as they rise through. Why Does Temperature Rise With Pressure.
From courses.lumenlearning.com
Phase Changes Physics Why Does Temperature Rise With Pressure We can understand this using an idea called the. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. The energy added as work during. Why Does Temperature Rise With Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Why Does Temperature Rise With Pressure But if the same data is used and the graph extended to the left, at what. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. The energy added as work during the compression of a gas leads to an increase in pressure. Why Does Temperature Rise With Pressure.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Why Does Temperature Rise With Pressure But if the same data is used and the graph extended to the left, at what. Learn more about this in this article. That is, the atmospheric gas exerts less. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather balloons get larger as they rise through. Why Does Temperature Rise With Pressure.
From www.youtube.com
Effects of Temperature and Pressure on Matter iKen iKen Edu iKen Why Does Temperature Rise With Pressure Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; But if the same data is used and the graph extended to the left, at what. That is, the atmospheric gas exerts less. The energy added as work during the compression of a gas leads to an. Why Does Temperature Rise With Pressure.
From byjus.com
what is Why Does Temperature Rise With Pressure This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; That is, the atmospheric gas exerts less. Learn more about this in this article. But if the same data. Why Does Temperature Rise With Pressure.
From socratic.org
Question 15820 Socratic Why Does Temperature Rise With Pressure We can understand this using an idea called the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? Increasing temperature increases the average kinetic energy (ke) and the average velocity of. Why Does Temperature Rise With Pressure.
From garciaspeausell.blogspot.com
Why Did the Temperature Rise to a Constant Temperature Instead of Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. But if the same data is used and the graph extended to the left, at what. That is, the atmospheric gas exerts less. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? This graph. Why Does Temperature Rise With Pressure.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Why Does Temperature Rise With Pressure Learn more about this in this article. We can understand this using an idea called the. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? If you had a way. Why Does Temperature Rise With Pressure.
From masterconceptsinchemistry.com
Why does volume gas increase as temperature increase? Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Why does increasing the temperature of a. Why Does Temperature Rise With Pressure.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Why Does Temperature Rise With Pressure That is, the atmospheric gas exerts less. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. In reality, most compression take place by reducing volume. We can understand this using an idea called the. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules. Why Does Temperature Rise With Pressure.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and Why Does Temperature Rise With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas. Why Does Temperature Rise With Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Why Does Temperature Rise With Pressure Learn more about this in this article. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. In reality, most compression take place by reducing volume. The energy added. Why Does Temperature Rise With Pressure.
From sciencenotes.org
Le Chatelier's Principle Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. In reality, most compression take place by reducing volume. Weather balloons get larger as. Why Does Temperature Rise With Pressure.
From www.eoas.ubc.ca
UBC ATSC 113 Layers in the Standard Atmosphere Why Does Temperature Rise With Pressure Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; But if the same data is used and the graph extended to the left, at what. In reality, most compression take place by reducing volume. The energy added as work during the compression of a gas leads. Why Does Temperature Rise With Pressure.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. But if the same data is used and the graph extended to the left, at what. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. We can understand. Why Does Temperature Rise With Pressure.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; That is, the atmospheric gas exerts less. Why does increasing the temperature of a gas increase the pressure. Why Does Temperature Rise With Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Why Does Temperature Rise With Pressure In reality, most compression take place by reducing volume. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Learn more about this in this article. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the. Why Does Temperature Rise With Pressure.
From slideplayer.com
Chapter 14 Gases. ppt download Why Does Temperature Rise With Pressure We can understand this using an idea called the. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. But if the same data is used and the graph extended to the left, at what. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the. Why Does Temperature Rise With Pressure.
From socratic.org
How does atmospheric pressure change with altitude? Socratic Why Does Temperature Rise With Pressure But if the same data is used and the graph extended to the left, at what. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. That is, the atmospheric gas exerts less. We can understand this using an idea called the. In reality, most compression take place. Why Does Temperature Rise With Pressure.
From gcsephysicsninja.com
11. Heating gas at a constant pressure Why Does Temperature Rise With Pressure Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? We can understand this using an idea called the. In reality, most compression take place by reducing volume. The energy added. Why Does Temperature Rise With Pressure.
From www.numerade.com
SOLVEDIndicate which diagram (1, 2, or 3) represents the volume of the Why Does Temperature Rise With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas. Why Does Temperature Rise With Pressure.
From ar.inspiredpencil.com
Normal Body Temperature Diagram Why Does Temperature Rise With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. But if the same data is used and the graph extended. Why Does Temperature Rise With Pressure.
From rofaeducationcentre.blogspot.com
[Kunci Jawaban] Bandingkan tekanan pada kedua puncak tersebut. Mengapa Why Does Temperature Rise With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? If you had a way to increase. Why Does Temperature Rise With Pressure.
From mungfali.com
Maxwell Boltzmann Distribution Temperature Why Does Temperature Rise With Pressure Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? Learn more about this in this article. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. We can understand this using an idea called the. That. Why Does Temperature Rise With Pressure.
From chem.libretexts.org
13.4 Effects of Temperature and Pressure on Solubility Chemistry Why Does Temperature Rise With Pressure Learn more about this in this article. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; We can understand this using an idea called the. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and. Why Does Temperature Rise With Pressure.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Why Does Temperature Rise With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. We can understand this using an idea called the. But if the same data is used and the graph extended to the left, at what. Why does increasing the temperature of a gas increase the pressure (when the. Why Does Temperature Rise With Pressure.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Why Does Temperature Rise With Pressure This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Learn more about this in this article. We can understand this using an idea called the. The energy added as. Why Does Temperature Rise With Pressure.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Why Does Temperature Rise With Pressure But if the same data is used and the graph extended to the left, at what. Learn more about this in this article. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas. Why Does Temperature Rise With Pressure.
From socratic.org
How do mountains affect temperature on both the windward side and the Why Does Temperature Rise With Pressure We can understand this using an idea called the. Why does increasing the temperature of a gas increase the pressure (when the volume is fixed)? Learn more about this in this article. But if the same data is used and the graph extended to the left, at what. That is, the atmospheric gas exerts less. Weather balloons get larger as. Why Does Temperature Rise With Pressure.
From garciaspeausell.blogspot.com
Why Did the Temperature Rise to a Constant Temperature Instead of Why Does Temperature Rise With Pressure Increasing temperature increases the average kinetic energy (ke) and the average velocity of the gas molecules resulting in more frequent and more forceful collisions which result in. Weather balloons get larger as they rise through the atmosphere to regions of lower pressure because the volume of the gas has increased; This graph tells us that as temperature increases, kinetic energy. Why Does Temperature Rise With Pressure.