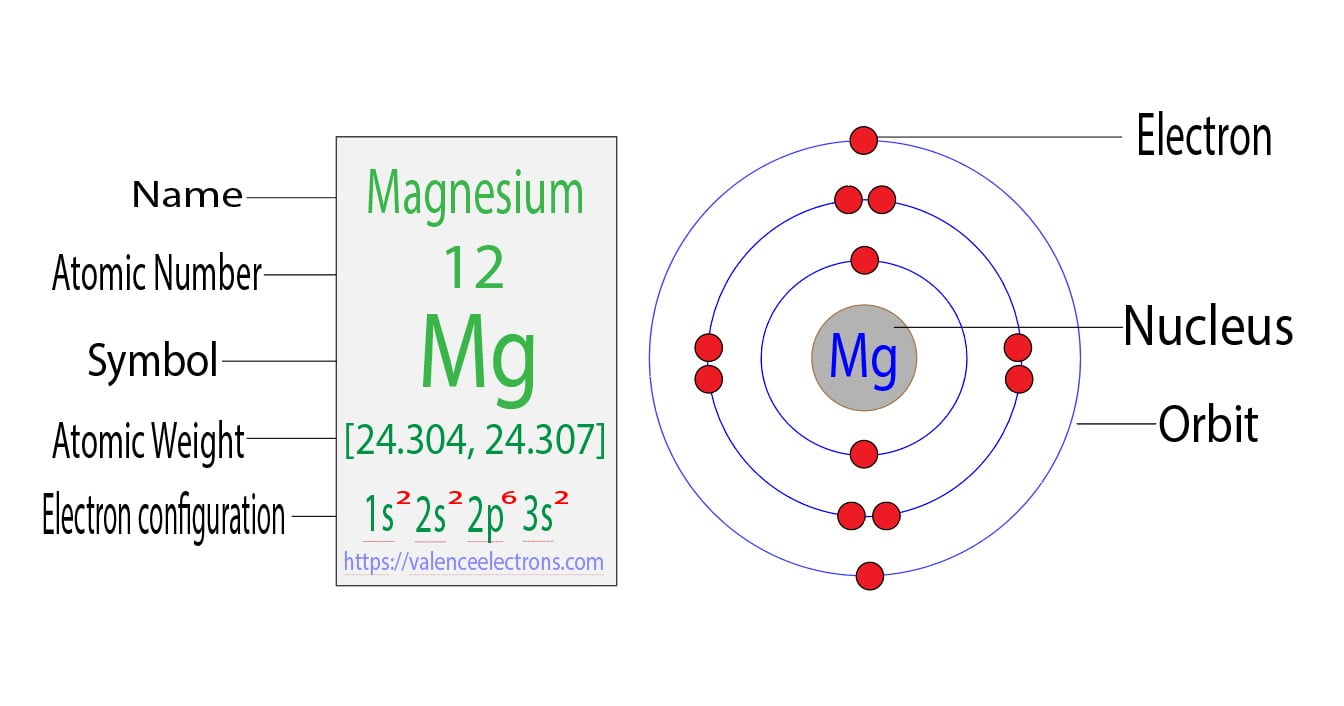
Does Magnesium Gain Or Lose Electrons . 1, 2 and 3, the number of electrons lost is the same as the group number. A sodium atom loses one electron to form a sodium. magnesium’s position in the periodic table (group 2) tells us that it is a metal. This is because it is in the second group of the periodic table, and elements in this group. magnesium’s position in the periodic table tells us that it is a metal. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. Metals form positive ions (cations). magnesium loses two electrons when reacting. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number.
from valenceelectrons.com
This is because it is in the second group of the periodic table, and elements in this group. Metals form positive ions (cations). magnesium’s position in the periodic table tells us that it is a metal. magnesium’s position in the periodic table (group 2) tells us that it is a metal. A magnesium atom must lose two electrons to have the same number. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. magnesium loses two electrons when reacting. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 1, 2 and 3, the number of electrons lost is the same as the group number. Metals form positive ions (cations).
Magnesium Electron Configuration Aufbau & Bohr Model
Does Magnesium Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium loses two electrons when reacting. Metals form positive ions (cations). magnesium’s position in the periodic table (group 2) tells us that it is a metal. Metals form positive ions (cations). magnesium’s position in the periodic table tells us that it is a metal. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. A magnesium atom must lose two electrons to have the same number. A sodium atom loses one electron to form a sodium. 1, 2 and 3, the number of electrons lost is the same as the group number. This is because it is in the second group of the periodic table, and elements in this group.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID270027 Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. 1, 2 and 3, the number of electrons lost is the same as the group number. Metals form positive ions (cations). magnesium loses two electrons when reacting. Metals form positive ions (cations). magnesium’s position in the periodic table (group 2) tells us that it is a. Does Magnesium Gain Or Lose Electrons.
From shaunmwilliams.com
Lecture 2 Presentation Does Magnesium Gain Or Lose Electrons This is because it is in the second group of the periodic table, and elements in this group. Metals form positive ions (cations). A sodium atom loses one electron to form a sodium. 1, 2 and 3, the number of electrons lost is the same as the group number. when forming ions, elements typically gain or lose the minimum. Does Magnesium Gain Or Lose Electrons.
From cetxkhtd.blob.core.windows.net
Magnesium Electrons To Gain at Stefania Neth blog Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. magnesium’s position in the periodic. Does Magnesium Gain Or Lose Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). 1, 2 and 3, the number of electrons lost is the same as the group number. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium’s position in the periodic table tells us that it is a metal. A magnesium atom must lose. Does Magnesium Gain Or Lose Electrons.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. This is because it is in the second group of the periodic table, and elements in this group. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium’s position in the periodic table (group 2) tells. Does Magnesium Gain Or Lose Electrons.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metals form positive ions (cations). A. Does Magnesium Gain Or Lose Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Does Magnesium Gain Or Lose Electrons 1, 2 and 3, the number of electrons lost is the same as the group number. This is because it is in the second group of the periodic table, and elements in this group. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium loses two electrons when. Does Magnesium Gain Or Lose Electrons.
From new-periodic11.blogspot.com
NEW MAGNESIUM PERIODIC TABLE PROTONS Periodic Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium’s position in the periodic table tells us that it is a metal. 1, 2 and 3, the number of electrons lost is the same as the. Does Magnesium Gain Or Lose Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Does Magnesium Gain Or Lose Electrons This is because it is in the second group of the periodic table, and elements in this group. A magnesium atom must lose two electrons to have the same number. magnesium loses two electrons when reacting. 1, 2 and 3, the number of electrons lost is the same as the group number. magnesium’s position in the periodic table. Does Magnesium Gain Or Lose Electrons.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). 1, 2 and 3, the number of electrons lost is the same as the group number. magnesium’s position in the periodic table tells us that it is a metal. Metals form positive ions (cations). atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the. Does Magnesium Gain Or Lose Electrons.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table (group 2) tells us that it is a metal. A magnesium atom must lose two electrons to have the same number. 1, 2 and 3, the number of electrons lost is the same as the group number. when forming ions, elements typically gain or lose the minimum number of electrons necessary to. Does Magnesium Gain Or Lose Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5581477 Does Magnesium Gain Or Lose Electrons magnesium loses two electrons when reacting. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metals form positive ions (cations). magnesium’s position in the periodic table (group 2) tells us that it is a metal. 1, 2 and 3, the number of electrons lost is the same. Does Magnesium Gain Or Lose Electrons.
From slideplayer.com
Chapter 6 Section ppt download Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table (group 2) tells us that it is a metal. This is because it is in the second group of the periodic table, and elements in this group. A sodium atom loses one electron to form a sodium. magnesium loses two electrons when reacting. magnesium’s position in the periodic table tells us. Does Magnesium Gain Or Lose Electrons.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Does Magnesium Gain Or Lose Electrons A sodium atom loses one electron to form a sodium. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. A magnesium atom must lose two electrons to have the same number. This is because it is in the second group of the periodic table, and elements in this group.. Does Magnesium Gain Or Lose Electrons.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table (group 2) tells us that it is a metal. This is because it is in the second group of the periodic table, and elements in this group. Metals form positive ions (cations). when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. . Does Magnesium Gain Or Lose Electrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). 1, 2 and 3, the number of electrons lost is the same as the group number. magnesium loses two electrons when reacting. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. This is because it is in the second group of the periodic. Does Magnesium Gain Or Lose Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). This is because it is in the second group of the periodic table, and elements in this group. magnesium loses two electrons when reacting. A sodium atom loses one electron to form a sodium. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the. Does Magnesium Gain Or Lose Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table tells us that it is a metal. This is because it is in the second group of the periodic table, and elements in this group. A magnesium atom must lose two electrons to have the same number. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the. Does Magnesium Gain Or Lose Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Does Magnesium Gain Or Lose Electrons 1, 2 and 3, the number of electrons lost is the same as the group number. magnesium’s position in the periodic table (group 2) tells us that it is a metal. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. A magnesium atom must lose two electrons to. Does Magnesium Gain Or Lose Electrons.
From www.slideserve.com
PPT CHEMICAL FORMULAS AND BONDING PowerPoint Presentation, free Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table tells us that it is a metal. magnesium’s position in the periodic table (group 2) tells us that it is a metal. Metals form positive ions (cations). atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. 1, 2 and. Does Magnesium Gain Or Lose Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. magnesium’s position in the periodic table tells us that it is a metal. Metals form positive ions (cations). magnesium loses two electrons when reacting. 1, 2 and 3, the number of electrons lost is the same as the group number. This is because it is in. Does Magnesium Gain Or Lose Electrons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium’s position in the periodic table tells us that it is a metal. magnesium’s position in the periodic table (group 2) tells us that it is. Does Magnesium Gain Or Lose Electrons.
From www.numerade.com
SOLVED 11. When it forms an ion, is magnesium likely to gain or lose Does Magnesium Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. This is because it is in the second group of the periodic table, and elements in this group. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble.. Does Magnesium Gain Or Lose Electrons.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Does Magnesium Gain Or Lose Electrons This is because it is in the second group of the periodic table, and elements in this group. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. A magnesium atom must lose two electrons to have the same number. atoms tend to lose, gain, or share some valance. Does Magnesium Gain Or Lose Electrons.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Does Magnesium Gain Or Lose Electrons A sodium atom loses one electron to form a sodium. Metals form positive ions (cations). magnesium’s position in the periodic table (group 2) tells us that it is a metal. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. magnesium loses two electrons when reacting.. Does Magnesium Gain Or Lose Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Does Magnesium Gain Or Lose Electrons A sodium atom loses one electron to form a sodium. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. Metals form positive ions (cations). when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium’s position. Does Magnesium Gain Or Lose Electrons.
From www.numerade.com
SOLVED According to the octet rule, a magnesium atom has a tendency to Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). magnesium loses two electrons when reacting. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. A sodium atom loses one electron to form a sodium. 1, 2 and 3, the number of electrons lost is the same as the group number.. Does Magnesium Gain Or Lose Electrons.
From userdatalicensures.z5.web.core.windows.net
Magnesium And Nitrogen Lewis Dot Structure Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table (group 2) tells us that it is a metal. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. magnesium loses two electrons when reacting. magnesium’s position in the periodic table tells us that it is a metal. Metals. Does Magnesium Gain Or Lose Electrons.
From www.slideserve.com
PPT Oxidation an atom loses one or more electrons . PowerPoint Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). magnesium’s position in the periodic table tells us that it is a metal. magnesium loses two electrons when reacting. A sodium atom loses one electron to form a sodium. magnesium’s position in the periodic table (group 2) tells us that it is a metal. Metals form positive ions (cations). when forming. Does Magnesium Gain Or Lose Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Does Magnesium Gain Or Lose Electrons A magnesium atom must lose two electrons to have the same number. magnesium’s position in the periodic table (group 2) tells us that it is a metal. A sodium atom loses one electron to form a sodium. magnesium loses two electrons when reacting. 1, 2 and 3, the number of electrons lost is the same as the group. Does Magnesium Gain Or Lose Electrons.
From slideplayer.com
IONS Big Idea Atoms can gain or lose electrons to form charged Does Magnesium Gain Or Lose Electrons This is because it is in the second group of the periodic table, and elements in this group. A sodium atom loses one electron to form a sodium. magnesium loses two electrons when reacting. Metals form positive ions (cations). Metals form positive ions (cations). when forming ions, elements typically gain or lose the minimum number of electrons necessary. Does Magnesium Gain Or Lose Electrons.
From thefitnessmanual.com
Does Magnesium Lose Or Gain Electrons TheFitnessManual Does Magnesium Gain Or Lose Electrons Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number. magnesium’s position in the periodic table (group 2) tells us that it is a metal. magnesium’s position in the periodic table tells us that it is a metal. when forming ions, elements typically gain or lose the minimum number of. Does Magnesium Gain Or Lose Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Does Magnesium Gain Or Lose Electrons magnesium’s position in the periodic table tells us that it is a metal. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble. Metals form positive ions (cations). when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full. Does Magnesium Gain Or Lose Electrons.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Does Magnesium Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium loses two electrons when reacting. This is because it is in the second group of the periodic table, and elements in this group. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the. Does Magnesium Gain Or Lose Electrons.
From cebkouad.blob.core.windows.net
Magnesium Electrons Cation at Sarah Scoggins blog Does Magnesium Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metals form positive ions (cations). A sodium atom loses one electron to form a sodium. magnesium’s position in the periodic table (group 2) tells us that it is a metal. This is because it is in the second group. Does Magnesium Gain Or Lose Electrons.