Calorimetry Exothermic Or Endothermic . If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. The chemical reactions in a candle flame give off heat. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. Adding heat helps sugar and salt dissolve in water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, dissolving a solution of ammonium nitrate in water is. By knowing the change in heat, it can be. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An endothermic reaction is when the system or reaction gains energy from its surroundings. Reactants + energy → products.
from pediaa.com
One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, dissolving a solution of ammonium nitrate in water is. Adding heat helps sugar and salt dissolve in water. For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The chemical reactions in a candle flame give off heat. Reactants + energy → products. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
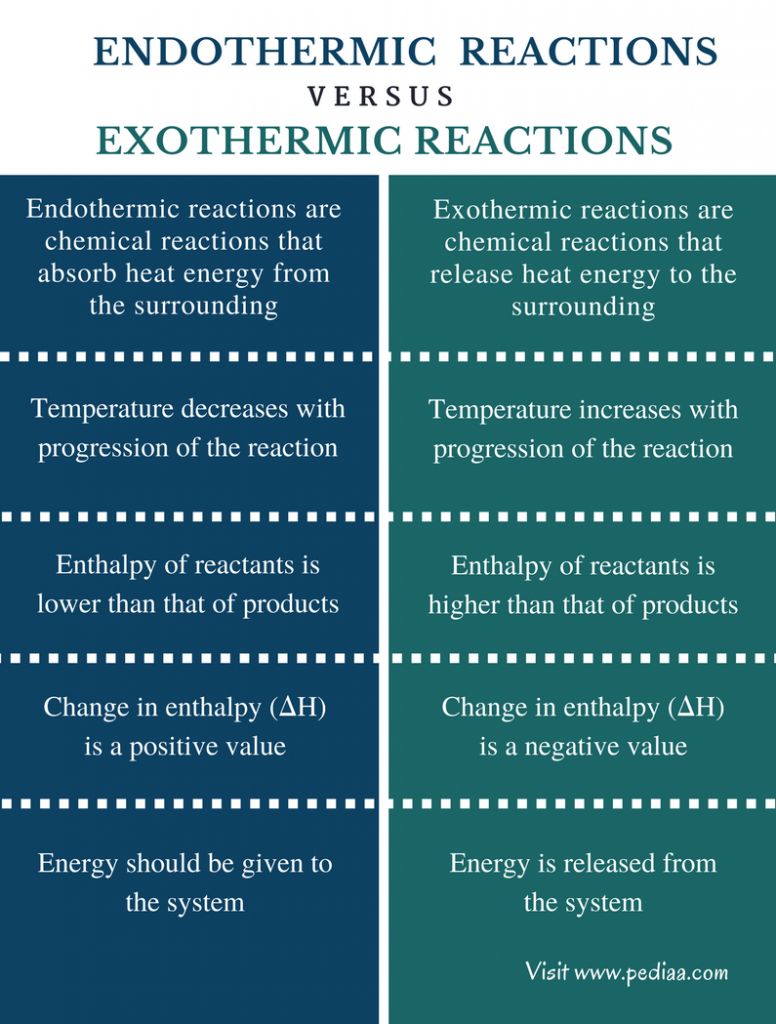
Difference Between Endothermic and Exothermic Reactions Definition
Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in heat, it can be. For example, dissolving a solution of ammonium nitrate in water is. For example, when an exothermic. Reactants + energy → products. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The chemical reactions in a candle flame give off heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An endothermic reaction is when the system or reaction gains energy from its surroundings. Adding heat helps sugar and salt dissolve in water.
From slideplayer.com
THERMODYNAMICS Intro & Calorimetry. ppt download Calorimetry Exothermic Or Endothermic The chemical reactions in a candle flame give off heat. Reactants + energy → products. For example, when an exothermic. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. If we run an exothermic reaction in solution in. Calorimetry Exothermic Or Endothermic.
From www.slideserve.com
PPT Laboratory 12 CALORIMETRY PowerPoint Presentation, free download Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An endothermic process absorbs heat from the surroundings, while an exothermic process. Calorimetry Exothermic Or Endothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Calorimetry Exothermic Or Endothermic For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. An endothermic reaction is when the system or reaction gains energy from its surroundings. By knowing the change in. Calorimetry Exothermic Or Endothermic.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Calorimetry Exothermic Or Endothermic Adding heat helps sugar and salt dissolve in water. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
Measuring Food Energy by Calorimetry ppt download Calorimetry Exothermic Or Endothermic Reactants + energy → products. An endothermic reaction is when the system or reaction gains energy from its surroundings. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Adding heat helps sugar and salt dissolve in water. For. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
The study of heat released or required by chemical reactions ppt download Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reactants + energy → products. For example, when an exothermic. By knowing the change in heat, it can be. An endothermic reaction is when the system or reaction gains energy from its surroundings. For example, when an exothermic reaction occurs in. Calorimetry Exothermic Or Endothermic.
From www.scribd.com
Summary of Endothermic & Exothermic Process Along With Calorimetry PDF Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use. Calorimetry Exothermic Or Endothermic.
From courses.lumenlearning.com
Calorimetry General Chemistry Calorimetry Exothermic Or Endothermic For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The chemical reactions in a candle flame give off heat. Adding heat helps sugar and salt dissolve in water. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, dissolving a solution of ammonium nitrate. Calorimetry Exothermic Or Endothermic.
From byjus.com
11. What is exothermic and endothermic reaction? Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, dissolving a solution of ammonium nitrate in water is. The chemical reactions in a candle flame give off heat. Calorimetry is the process of measuring the amount of heat. Calorimetry Exothermic Or Endothermic.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, dissolving a solution of ammonium nitrate in water is. Adding heat helps sugar and salt dissolve in. Calorimetry Exothermic Or Endothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Calorimetry Exothermic Or Endothermic By knowing the change in heat, it can be. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reactants + energy → products. Adding heat helps sugar and salt dissolve in. Calorimetry Exothermic Or Endothermic.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the amount of heat involved in a. Calorimetry Exothermic Or Endothermic.
From mungfali.com
Exothermic Vs Endothermic Reaction Graph Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Adding heat helps sugar and salt dissolve in water. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. An endothermic reaction is when the system or. Calorimetry Exothermic Or Endothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Calorimetry Exothermic Or Endothermic For example, when an exothermic. For example, dissolving a solution of ammonium nitrate in water is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. If we run an exothermic reaction in solution. Calorimetry Exothermic Or Endothermic.
From stock.adobe.com
Endothermic Vs Exothermic Reactions Infographic Diagram showing a Calorimetry Exothermic Or Endothermic Reactants + energy → products. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. An endothermic reaction is when the system or reaction gains energy from its surroundings. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to. Calorimetry Exothermic Or Endothermic.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Exothermic Or Endothermic The chemical reactions in a candle flame give off heat. For example, dissolving a solution of ammonium nitrate in water is. By knowing the change in heat, it can be. An endothermic reaction is when the system or reaction gains energy from its surroundings. Reactants + energy → products. An endothermic process absorbs heat from the surroundings, while an exothermic. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
Exothermic or Endothermic? ppt download Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in. Calorimetry Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Calorimetry Exothermic Or Endothermic Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. A calorimeter is a device used to measure the amount of heat involved in. Calorimetry Exothermic Or Endothermic.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. If we run an exothermic reaction in solution in a calorimeter, the heat produced. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
Exothermic or Endothermic? ppt download Calorimetry Exothermic Or Endothermic For example, when an exothermic. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. Reactants + energy → products. One technique we can. Calorimetry Exothermic Or Endothermic.
From www.numerade.com
SOLVED Draw the typical differential scanning calorimetry thermogram Calorimetry Exothermic Or Endothermic Adding heat helps sugar and salt dissolve in water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in heat, it can be. An endothermic reaction is when the system or reaction gains energy from its surroundings. For example, dissolving a solution of ammonium nitrate in water is.. Calorimetry Exothermic Or Endothermic.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Calorimetry Exothermic Or Endothermic By knowing the change in heat, it can be. For example, when an exothermic. The chemical reactions in a candle flame give off heat. An endothermic reaction is when the system or reaction gains energy from its surroundings. For example, dissolving a solution of ammonium nitrate in water is. A calorimeter is a device used to measure the amount of. Calorimetry Exothermic Or Endothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Calorimetry Exothermic Or Endothermic By knowing the change in heat, it can be. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic. The chemical reactions in a candle flame give off heat. One technique we can use to measure. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
Calorimetry. ppt download Calorimetry Exothermic Or Endothermic If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is trapped in the calorimeter and increases the temperature of the solution. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry Exothermic Or Endothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Calorimetry Exothermic Or Endothermic An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If we. Calorimetry Exothermic Or Endothermic.
From chem.libretexts.org
Differential Scanning Calorimetry Chemistry LibreTexts Calorimetry Exothermic Or Endothermic One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. Calorimetry Exothermic Or Endothermic.
From slideplayer.com
Chemical Energy and Calorimetry ppt download Calorimetry Exothermic Or Endothermic Adding heat helps sugar and salt dissolve in water. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The chemical reactions in a candle flame give off heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, dissolving. Calorimetry Exothermic Or Endothermic.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Calorimetry Exothermic Or Endothermic Adding heat helps sugar and salt dissolve in water. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in heat, it can be. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into the surroundings. If we run an exothermic reaction. Calorimetry Exothermic Or Endothermic.
From sciencenotes.org
Exothermic Reactions Definition and Examples Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Adding heat helps sugar and salt dissolve in water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. If we run an exothermic reaction in solution in a calorimeter, the heat produced by. Calorimetry Exothermic Or Endothermic.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Calorimetry Exothermic Or Endothermic One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. By knowing the change in heat, it can be. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. An endothermic reaction is when the system or reaction gains energy from. Calorimetry Exothermic Or Endothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Calorimetry Exothermic Or Endothermic A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, dissolving a solution of ammonium nitrate in water is. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. If we run an exothermic reaction in solution in a calorimeter, the heat. Calorimetry Exothermic Or Endothermic.
From www.slideserve.com
PPT Laboratory 12 CALORIMETRY PowerPoint Presentation, free download Calorimetry Exothermic Or Endothermic One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, dissolving a solution of ammonium nitrate in water is. By knowing the change in heat, it can be. If we run an exothermic reaction in solution in a calorimeter, the heat produced by the reaction is. Calorimetry Exothermic Or Endothermic.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Calorimetry Exothermic Or Endothermic One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Reactants + energy → products. By knowing the change in heat, it can be. Adding heat helps sugar and salt dissolve in water. A calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Exothermic Or Endothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Calorimetry Exothermic Or Endothermic Adding heat helps sugar and salt dissolve in water. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The chemical reactions in a candle flame give off heat. Reactants + energy → products.. Calorimetry Exothermic Or Endothermic.
From www.researchgate.net
Exothermic curves from differential scanning calorimetry (DSC) analysis Calorimetry Exothermic Or Endothermic Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in heat, it can be. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. An endothermic process absorbs heat from the surroundings, while an exothermic process releases heat into. Calorimetry Exothermic Or Endothermic.