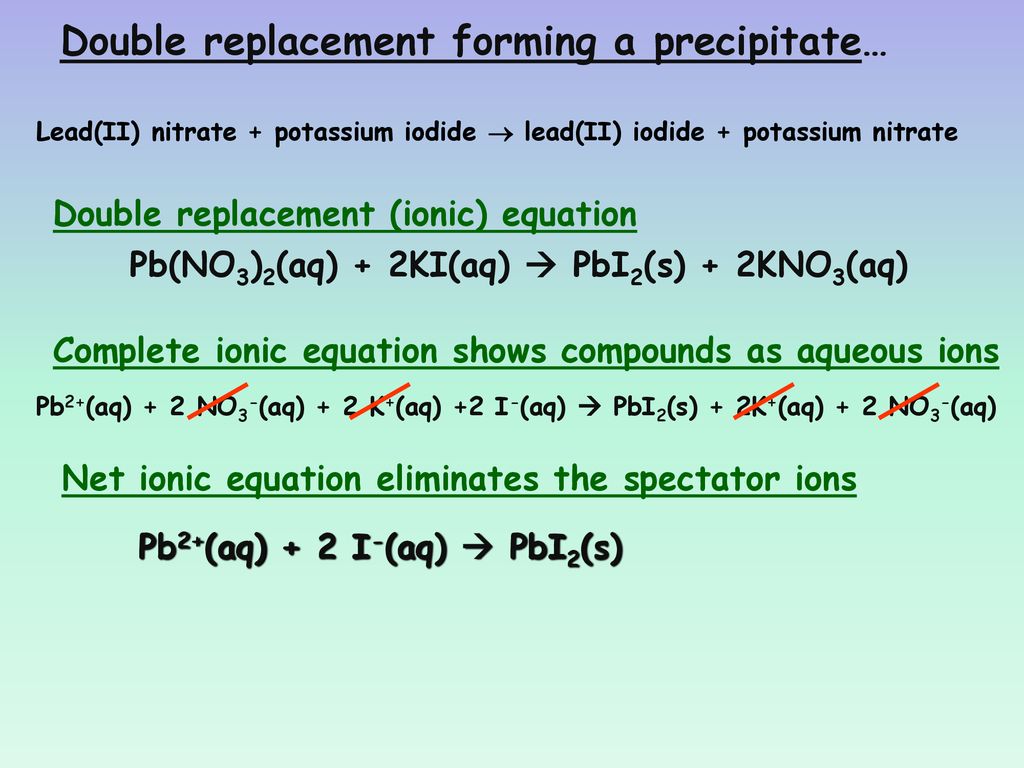
Lead (Ii) Nitrate + Lithium Chloride . Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. The balanced equation will be calculated along with the solubility states,. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. You could use things like. To do this experiment the materials. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. Binary ionic compounds are between a metal and nonmetal. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. An example of a precipitation. Here’s the best way to solve it.
from klarhocmw.blob.core.windows.net
An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. An example of a precipitation. Binary ionic compounds are between a metal and nonmetal. You could use things like. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. The balanced equation will be calculated along with the solubility states,.
Lead(Ii) Nitrate Sodium Bromide Precipitate at Micheal Malloy blog
Lead (Ii) Nitrate + Lithium Chloride Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. An example of a precipitation. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. To do this experiment the materials. The balanced equation will be calculated along with the solubility states,. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. You could use things like. Binary ionic compounds are between a metal and nonmetal. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Here’s the best way to solve it. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution.
From www.mingyuanchemical.com
Lead(II) nitrate 10099748 Lead (Ii) Nitrate + Lithium Chloride Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Binary ionic compounds. Lead (Ii) Nitrate + Lithium Chloride.
From www.numerade.com
SOLVED Write the correct net ionic equation for the reaction of Lead (Ii) Nitrate + Lithium Chloride An example of a precipitation. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You could use things like. Lead(ii) chloride, a white precipitate, is formed by. Lead (Ii) Nitrate + Lithium Chloride.
From www.abmstore.my
Lead II Nitrate AR / Plumbum II Nitrat Systerm (500g) AbmStore.my Lead (Ii) Nitrate + Lithium Chloride Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. Here’s the best way to solve it. Binary ionic compounds are between a metal and nonmetal. An experiment that produces lead. Lead (Ii) Nitrate + Lithium Chloride.
From www.carolina.com
Lead Nitrate, Crystal, Reagent Grade, 500 g Lead (Ii) Nitrate + Lithium Chloride You could use things like. To do this experiment the materials. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. Binary ionic compounds are between a metal and nonmetal. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in. Lead (Ii) Nitrate + Lithium Chloride.
From www.carolina.com
Lead (II) Nitrate ChemCapsule, Laboratory Grade, Pack of 5 Lead (Ii) Nitrate + Lithium Chloride The balanced equation will be calculated along with the solubility states,. Enter an equation of an ionic chemical equation and press the balance button. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Binary ionic compounds are between a metal and nonmetal.. Lead (Ii) Nitrate + Lithium Chloride.
From favpng.com
Lead(II) Nitrate Chemistry Chemical Compound, PNG, 1100x1100px, Leadii Lead (Ii) Nitrate + Lithium Chloride The balanced equation will be calculated along with the solubility states,. To do this experiment the materials. Enter an equation of an ionic chemical equation and press the balance button. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Here’s the best. Lead (Ii) Nitrate + Lithium Chloride.
From www.carolina.com
Lead (II) Nitrate Carolina Biological Supply Lead (Ii) Nitrate + Lithium Chloride Enter an equation of an ionic chemical equation and press the balance button. You could use things like. The balanced equation will be calculated along with the solubility states,. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Lead(ii) chloride, a white. Lead (Ii) Nitrate + Lithium Chloride.
From www.chemryt.com
img/p/51LEADNITRATE.png Lead (Ii) Nitrate + Lithium Chloride An example of a precipitation. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. You could use things like. To do this experiment the materials. The balanced equation will be calculated along with the solubility states,. Lead(ii) chloride can be made as. Lead (Ii) Nitrate + Lithium Chloride.
From molekula.com
Purchase Lead (II) nitrate [10099748] online • Catalog • Molekula Group Lead (Ii) Nitrate + Lithium Chloride This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated. Lead (Ii) Nitrate + Lithium Chloride.
From www.alibaba.com
Super High Purity 99.0lead Ii Nitrate Msds,Cas10099748 Buy Lead Lead (Ii) Nitrate + Lithium Chloride Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. This does not mean there are two atoms, but two types of atoms, so al 2 s 3. Lead (Ii) Nitrate + Lithium Chloride.
From www.walmart.com
1M Lead (II) Nitrate Solution, 500mL The Curated Chemical Collection Lead (Ii) Nitrate + Lithium Chloride To do this experiment the materials. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. An example of a precipitation. A. Lead (Ii) Nitrate + Lithium Chloride.
From www.indiamart.com
Lead Nitrate Powder at Rs 190/kg Pb(NO3)2 in Thane ID 8389650197 Lead (Ii) Nitrate + Lithium Chloride Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride. Lead (Ii) Nitrate + Lithium Chloride.
From www.labvalley.com
Lead (II) nitrate 99 AR.grade (500 กรัม/ขวด) ยี่ห้อ KemAus, Australia Lead (Ii) Nitrate + Lithium Chloride An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Binary ionic compounds are between a metal and nonmetal. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. Enter an equation of. Lead (Ii) Nitrate + Lithium Chloride.
From www.sigmaaldrich.id
LEAD(II) NITRATE, 99+, A.C.S. REAGENT Merck Life Science Indonesia Lead (Ii) Nitrate + Lithium Chloride Binary ionic compounds are between a metal and nonmetal. You could use things like. An example of a precipitation. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify. Lead (Ii) Nitrate + Lithium Chloride.
From klarhocmw.blob.core.windows.net
Lead(Ii) Nitrate Sodium Bromide Precipitate at Micheal Malloy blog Lead (Ii) Nitrate + Lithium Chloride To do this experiment the materials. Binary ionic compounds are between a metal and nonmetal. Here’s the best way to solve it. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. An example of a precipitation. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to. Lead (Ii) Nitrate + Lithium Chloride.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead (Ii) Nitrate + Lithium Chloride A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Enter an equation of an ionic chemical equation and press the balance button. You could use things like. Binary. Lead (Ii) Nitrate + Lithium Chloride.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead (Ii) Nitrate + Lithium Chloride This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric. Lead (Ii) Nitrate + Lithium Chloride.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead (Ii) Nitrate + Lithium Chloride An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. To do this experiment the materials. Lead(ii) chloride, a. Lead (Ii) Nitrate + Lithium Chloride.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii Lead (Ii) Nitrate + Lithium Chloride This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. A vivid. Lead (Ii) Nitrate + Lithium Chloride.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead (Ii) Nitrate + Lithium Chloride Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid. Lead (Ii) Nitrate + Lithium Chloride.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead (Ii) Nitrate + Lithium Chloride Here’s the best way to solve it. The balanced equation will be calculated along with the solubility states,. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. You could use things like. Binary ionic compounds are between a metal and nonmetal. This does not mean there are two atoms, but two types. Lead (Ii) Nitrate + Lithium Chloride.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Lead (Ii) Nitrate + Lithium Chloride Binary ionic compounds are between a metal and nonmetal. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Here’s the best way to solve it. This does not mean there are two atoms, but two types of atoms, so al 2 s. Lead (Ii) Nitrate + Lithium Chloride.
From www.ceramic-glazes.com
Lead(II) nitrate is used in the manufacture of matches and special Lead (Ii) Nitrate + Lithium Chloride Here’s the best way to solve it. To do this experiment the materials. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. You could use things like. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in. Lead (Ii) Nitrate + Lithium Chloride.
From www.southernbiological.com
Lead (II) Nitrate, 0.5M, Aqueous, Laboratory Grade Southern Biological Lead (Ii) Nitrate + Lithium Chloride An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. Binary ionic compounds are between a metal and nonmetal. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. Here’s the best way to solve. Lead (Ii) Nitrate + Lithium Chloride.
From klaalfdoh.blob.core.windows.net
Lead (Ii) Nitrate Reacts With Sodium Phosphate at James McCord blog Lead (Ii) Nitrate + Lithium Chloride A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. The balanced equation will be calculated along with the solubility states,. To do this experiment the materials. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. This does. Lead (Ii) Nitrate + Lithium Chloride.
From lab.honeywell.com
Lead(II) nitrate 228621 Honeywell Research Chemicals Lead (Ii) Nitrate + Lithium Chloride A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. You could use things like. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the solubility states,. To do this experiment the materials. Here’s. Lead (Ii) Nitrate + Lithium Chloride.
From apcpure.com
Lead (II) Nitrate 99 ACS 250gm APC Pure Lead (Ii) Nitrate + Lithium Chloride To do this experiment the materials. Here’s the best way to solve it. The balanced equation will be calculated along with the solubility states,. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. You could use things like. Enter an equation of an ionic chemical equation and press. Lead (Ii) Nitrate + Lithium Chloride.
From sincerechemical01.en.made-in-china.com
High Pure Factory 98 Chemical Grade Lead (II) Nitrate China Lead (II Lead (Ii) Nitrate + Lithium Chloride A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. The balanced equation will be calculated along with the solubility states,. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. Binary ionic compounds are between a metal. Lead (Ii) Nitrate + Lithium Chloride.
From www.carolina.com
Lead (II) Nitrate Solution, 0.5 M Aqueous, Laboratory Grade, 500 mL Lead (Ii) Nitrate + Lithium Chloride Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. To do this experiment the materials. An example of a precipitation. A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. Lead(ii) chloride can be made as a white precipitate by adding a solution containing. Lead (Ii) Nitrate + Lithium Chloride.
From www.mosinterchem.com.cn
Lead II nitrate CAS 10099748 Chemicals supplier from China Lead (Ii) Nitrate + Lithium Chloride Binary ionic compounds are between a metal and nonmetal. The balanced equation will be calculated along with the solubility states,. This does not mean there are two atoms, but two types of atoms, so al 2 s 3 is a binary. An example of a precipitation. You could use things like. Here’s the best way to solve it. An experiment. Lead (Ii) Nitrate + Lithium Chloride.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Lead (Ii) Nitrate + Lithium Chloride You could use things like. Binary ionic compounds are between a metal and nonmetal. Lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. To do this experiment the materials. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. A precipitation. Lead (Ii) Nitrate + Lithium Chloride.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead (Ii) Nitrate + Lithium Chloride Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. To do this experiment the materials. This does not mean there are two. Lead (Ii) Nitrate + Lithium Chloride.
From www.youtube.com
How to Balance Pb(NO3)2 + LiCl = PbCl2 + LiNO3 Lead (II) nitrate Lead (Ii) Nitrate + Lithium Chloride To do this experiment the materials. You could use things like. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead. Enter an equation of an ionic chemical equation and press the balance button. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate. Lead (Ii) Nitrate + Lithium Chloride.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Lead (Ii) Nitrate + Lithium Chloride A precipitation reaction is a reaction that yields an insoluble product—a precipitate —when two solutions are mixed. You could use things like. An example of a precipitation. To do this experiment the materials. Write the balanced molecular equation for the reaction between lead (ii) nitrate and lithium chloride to identify the. Here’s the best way to solve it. Binary ionic. Lead (Ii) Nitrate + Lithium Chloride.
From www.sciencephoto.com
Lead (II) nitrate, Pb(NO3)2 Stock Image C030/7163 Science Photo Lead (Ii) Nitrate + Lithium Chloride Binary ionic compounds are between a metal and nonmetal. An experiment that produces lead (ll) chloride is combining lead (ll) nitrate and lithium chloride would be to mix the two compounds together in an aqueous solution. A vivid example of precipitation is observed when solutions of potassium iodide and lead nitrate are mixed, resulting in the formation of solid lead.. Lead (Ii) Nitrate + Lithium Chloride.