Zinc Carbonate Reaction With Hydrochloric Acid . Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 + 2hcl → zncl2 + co2 + h2o. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The chemical reaction is as below. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Calcium carbonate occurs naturally as chalk, limestone and marble. Znco3 → zno + co2. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. 2hcl + zn → zncl 2 + h 2.
from www.numerade.com
Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. 2hcl + zn → zncl 2 + h 2. Znco3 + 2hcl → zncl2 + co2 + h2o. Calcium carbonate occurs naturally as chalk, limestone and marble. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Znco3 → zno + co2. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide.
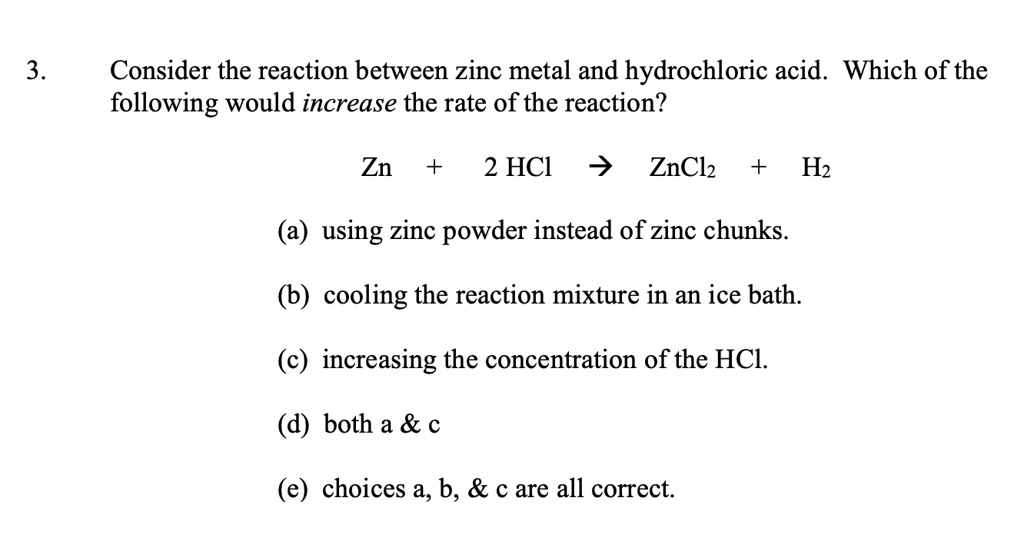
SOLVED Consider the reaction between zinc metal and hydrochloric acid
Zinc Carbonate Reaction With Hydrochloric Acid 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. 2hcl + zn → zncl 2 + h 2. The chemical reaction is as below. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Znco3 + 2hcl → zncl2 + co2 + h2o. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Calcium carbonate occurs naturally as chalk, limestone and marble. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Znco3 → zno + co2. Hydrochloric acid + zinc → zinc chloride + hydrogen.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Carbonate Reaction With Hydrochloric Acid Znco3 → zno + co2. Znco3 + 2hcl → zncl2 + co2 + h2o. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Calcium carbonate occurs naturally as chalk, limestone and marble. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and.. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0223 Zinc Carbonate Reaction With Hydrochloric Acid Znco3 + 2hcl → zncl2 + co2 + h2o. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Name the products of the reaction between. Zinc Carbonate Reaction With Hydrochloric Acid.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Znco3 → zno + co2. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 2hcl + zn → zncl 2 + h 2. Calcium carbonate occurs naturally. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Zinc and 6 M Hydrochloric Acid Reaction YouTube Zinc Carbonate Reaction With Hydrochloric Acid Znco3 → zno + co2. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The hydrogen. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Carbonate Reaction With Hydrochloric Acid For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 → zno + co2. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Znco3 + 2hcl → zncl2 + co2 + h2o. 2hcl + zn → zncl 2 + h 2. Zinc carbonate undergoes decomposition. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid Zinc Carbonate Reaction With Hydrochloric Acid Znco3 → zno + co2. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Znco3 + 2hcl → zncl2 + co2 + h2o. Zinc carbonate undergoes decomposition forming zinc oxide. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction Zinc Carbonate Reaction With Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Znco3 + 2hcl → zncl2 + co2 + h2o. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Calcium carbonate occurs naturally as chalk, limestone and marble. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol. Zinc Carbonate Reaction With Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Carbonate Reaction With Hydrochloric Acid The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Calcium carbonate occurs naturally as chalk, limestone and marble. Znco3 → zno + co2. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Znco3 + 2hcl → zncl2 + co2 + h2o. 2hcl + zn → zncl 2 + h 2. Consider the reaction between. Zinc Carbonate Reaction With Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Carbonate Reaction With Hydrochloric Acid The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. 2hcl + zn → zncl 2 + h 2. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. For. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. 2hcl + zn → zncl 2 + h 2. Hydrochloric acid + zinc → zinc chloride + hydrogen. Calcium carbonate occurs naturally as chalk, limestone and marble. Zinc carbonate reacts with. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.toppr.com
Zinc and hydrochloric acid react according to the reaction Zn(s Zinc Carbonate Reaction With Hydrochloric Acid Znco3 + 2hcl → zncl2 + co2 + h2o. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. 2hcl + zn → zncl 2 + h 2. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. The hydrogen causes bubbling during the reaction, and can be detected. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc Carbonate Reaction With Hydrochloric Acid Hydrochloric acid + zinc → zinc chloride + hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 + 2hcl → zncl2 + co2 + h2o. Znco3 → zno + co2. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Zinc carbonate. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.chegg.com
Solved Solid zinc(II) carbonate reacts with us Hydrochloric Zinc Carbonate Reaction With Hydrochloric Acid \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Name the products of the reaction between. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 2hcl + zn → zncl 2 + h 2. Calcium carbonate occurs naturally as chalk, limestone and marble. The chemical reaction is as below. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 → zno + co2. Znco3 + 2hcl → zncl2 + co2 + h2o. The chemical reaction is as below. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Calcium carbonate occurs naturally. Zinc Carbonate Reaction With Hydrochloric Acid.
From brainly.in
What are the gases liberated when the dilute hydrochloric acid react Zinc Carbonate Reaction With Hydrochloric Acid Calcium carbonate occurs naturally as chalk, limestone and marble. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. The chemical reaction is as below. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Zinc carbonate reacts with acids like. Zinc Carbonate Reaction With Hydrochloric Acid.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Carbonate Reaction With Hydrochloric Acid \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. The chemical reaction is as below. Calcium carbonate occurs naturally as chalk, limestone and marble. Hydrochloric acid + zinc → zinc chloride + hydrogen. Name the products of the reaction between zinc carbonate and sulfuric acid, and write. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Chemical Reaction zinc and hydrochloric acid YouTube Zinc Carbonate Reaction With Hydrochloric Acid Calcium carbonate occurs naturally as chalk, limestone and marble. Znco3 → zno + co2. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid + zinc → zinc chloride + hydrogen. 2hcl + zn → zncl 2 + h 2. The chemical reaction is as below. Zinc carbonate reacts with acids like hydrochloric acid. Zinc Carbonate Reaction With Hydrochloric Acid.
From jaylongokelawrence.blogspot.com
Zinc Reacts With Hydrochloric Acid Zinc Carbonate Reaction With Hydrochloric Acid Znco3 → zno + co2. Hydrochloric acid + zinc → zinc chloride + hydrogen. 2hcl + zn → zncl 2 + h 2. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Name the products of the reaction between zinc carbonate and sulfuric. Zinc Carbonate Reaction With Hydrochloric Acid.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Carbonate Reaction With Hydrochloric Acid 2hcl + zn → zncl 2 + h 2. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. Calcium carbonate occurs naturally as chalk,. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc Carbonate Reaction With Hydrochloric Acid The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Znco3 + 2hcl → zncl2 + co2 + h2o. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. The chemical. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. 2hcl + zn → zncl 2 + h 2. Calcium carbonate occurs naturally as chalk, limestone and marble. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Znco3 → zno + co2. Name. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc Carbonate Reaction With Hydrochloric Acid Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Calcium carbonate occurs naturally as chalk, limestone and marble. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. The hydrogen causes bubbling during. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.slideshare.net
Acids And Bases Zinc Carbonate Reaction With Hydrochloric Acid Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Znco3 + 2hcl → zncl2 + co2 + h2o. Znco3 → zno + co2.. Zinc Carbonate Reaction With Hydrochloric Acid.
From fineartamerica.com
Zinc Reacting With Hydrochloric Acid Photograph by Martyn F. Chillmaid Zinc Carbonate Reaction With Hydrochloric Acid Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. Znco3 + 2hcl → zncl2 + co2 + h2o. Calcium carbonate occurs naturally as chalk, limestone and marble. Znco3 → zno + co2. \[\ce{zn(s) +. Zinc Carbonate Reaction With Hydrochloric Acid.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc Carbonate Reaction With Hydrochloric Acid 2hcl + zn → zncl 2 + h 2. The chemical reaction is as below. Znco3 + 2hcl → zncl2 + co2 + h2o. Calcium carbonate occurs naturally as chalk, limestone and marble. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. The hydrogen causes bubbling during the reaction, and. Zinc Carbonate Reaction With Hydrochloric Acid.
From slideplayer.com
Types of Chemical Reactions Ch ppt download Zinc Carbonate Reaction With Hydrochloric Acid The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. The chemical reaction is as below. Calcium carbonate occurs naturally as chalk, limestone and marble. Name the products of the reaction between zinc. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.coursehero.com
[Solved] Zinc reacts with hydrochloric acid according to this reaction Zinc Carbonate Reaction With Hydrochloric Acid The chemical reaction is as below. Hydrochloric acid + zinc → zinc chloride + hydrogen. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Znco3 + 2hcl → zncl2 + co2 + h2o. Zinc carbonate reacts with acids like hydrochloric acid to form zinc. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Zinc Carbonate Reaction With Hydrochloric Acid Calcium carbonate occurs naturally as chalk, limestone and marble. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. For example, zinc metal reacts with. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc Carbonate Reaction With Hydrochloric Acid \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. 2hcl + zn → zncl 2 + h 2. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. The chemical reaction is as below. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Zinc Carbonate Reaction With Hydrochloric Acid Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide. The chemical reaction is as below. Znco3 → zno + co2. Consider the reaction between zinc carbonate (znco3), and hydrochloric acid (hcl) to form zinc chloride (zncl2), water (h2o) and. Name the products of the reaction between zinc carbonate and sulfuric acid, and write. Zinc Carbonate Reaction With Hydrochloric Acid.
From dxorsdznx.blob.core.windows.net
Zinc And Hydrochloric Acid Net Ionic Equation at Kristine Murray blog Zinc Carbonate Reaction With Hydrochloric Acid Calcium carbonate occurs naturally as chalk, limestone and marble. Znco3 → zno + co2. Hydrochloric acid + zinc → zinc chloride + hydrogen. Znco3 + 2hcl → zncl2 + co2 + h2o. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The hydrogen causes bubbling during the reaction, and can be detected using a burning. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Zinc Carbonate Reaction With Hydrochloric Acid Calcium carbonate occurs naturally as chalk, limestone and marble. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Znco3 → zno + co2. The chemical reaction is as. Zinc Carbonate Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Zinc Carbonate Reaction With Hydrochloric Acid Znco3 → zno + co2. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Calcium carbonate occurs naturally as chalk, limestone and marble. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced symbol equation for the reaction. The. Zinc Carbonate Reaction With Hydrochloric Acid.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Zinc Carbonate Reaction With Hydrochloric Acid Znco3 + 2hcl → zncl2 + co2 + h2o. The chemical reaction is as below. Znco3 → zno + co2. The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Name the products of the reaction between zinc carbonate and sulfuric acid, and write a balanced. Zinc Carbonate Reaction With Hydrochloric Acid.