Fuel Cells In Acidic Electrolyte . A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Fuel cells have an important advantage over all. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity.
from anthropology.iresearchnet.com
Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. Fuel cells have an important advantage over all. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They are typically used in modules of 400 kw or. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions.
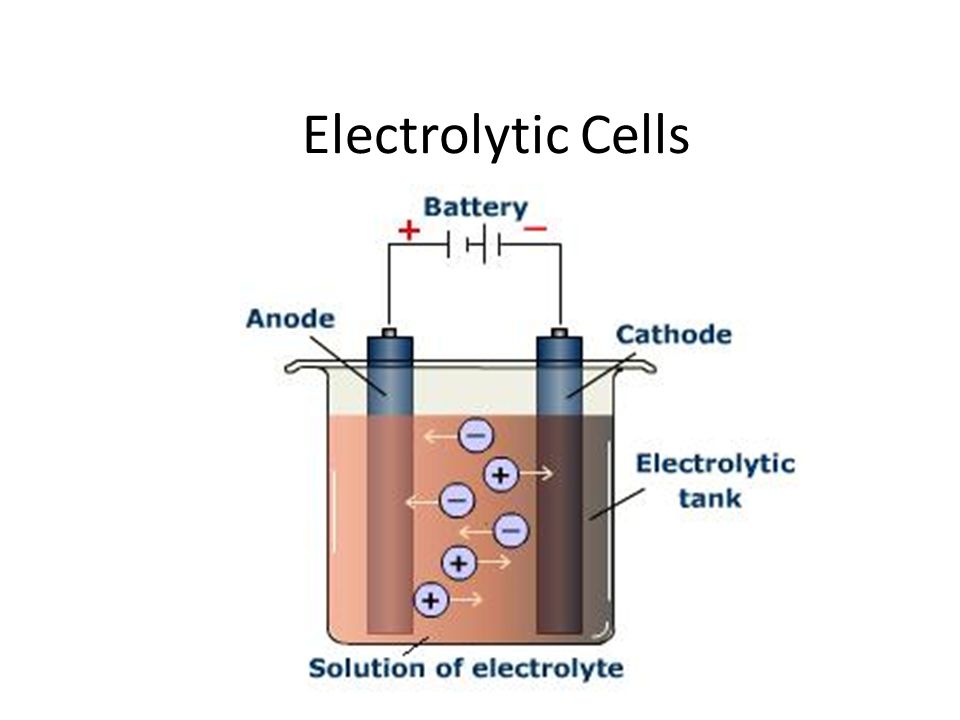
Tool Parts ordinary electrolytic cell redox reaction electrolytic cell
Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. Fuel cells have an important advantage over all. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange.
From www.youtube.com
Alkaline Fuel Cells (AFCs), Introduction, Principle, Advantages, Uses Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They are typically used in modules of 400 kw or. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or. Fuel Cells In Acidic Electrolyte.
From ar.inspiredpencil.com
Reaction Of Water Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant. Fuel Cells In Acidic Electrolyte.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Fuel Cells In Acidic Electrolyte Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. Fuel cells have an important advantage over all. This review discusses the history, fundamentals, and. Fuel Cells In Acidic Electrolyte.
From mcduffqws.blogspot.com
Direct Methanol Fuel Cell Mcduffqws Fuel Cells In Acidic Electrolyte Fuel cells have an important advantage over all. They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a. Fuel Cells In Acidic Electrolyte.
From www.dailymail.co.uk
easyJet unveils hybrid plane powered by BATTERIES and serves waste Fuel Cells In Acidic Electrolyte They are typically used in modules of 400 kw or. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A battery is a contained unit that produces electricity, whereas a. Fuel Cells In Acidic Electrolyte.
From www.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. Fuel cells have an important advantage over all. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held. Fuel Cells In Acidic Electrolyte.
From www.alamy.com
Phosphoric Acid fuel cell structure. Education technology info graphic Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They are typically used in modules of 400 kw or. Fuel cells have an important advantage over all. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and. Fuel Cells In Acidic Electrolyte.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. Phosphoric acid fuel cells use a. Fuel Cells In Acidic Electrolyte.
From anthropology.iresearchnet.com
Tool Parts ordinary electrolytic cell redox reaction electrolytic cell Fuel Cells In Acidic Electrolyte They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. A battery is a contained unit that produces electricity, whereas a fuel. Fuel Cells In Acidic Electrolyte.
From www.res.titech.ac.jp
Polyelectrolyte membrane for solidalkaline fuel cell Design and Fuel Cells In Acidic Electrolyte This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Fuel cells have an important advantage over all. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. The phosphoric acid fuel cell. Fuel Cells In Acidic Electrolyte.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Fuel Cells In Acidic Electrolyte They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Fuel cells have an important advantage over all. The phosphoric acid fuel. Fuel Cells In Acidic Electrolyte.
From revisechemistry.uk
Organic chemistry (chemistry only) OCR Gateway C6 revisechemistry.uk Fuel Cells In Acidic Electrolyte Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. They are typically used in modules. Fuel Cells In Acidic Electrolyte.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育 Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They are typically used in modules of 400 kw or. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Phosphoric acid fuel. Fuel Cells In Acidic Electrolyte.
From metabolicprocesses-ethanol.weebly.com
Ethanol Fuel Ethanol Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use. Fuel Cells In Acidic Electrolyte.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A battery is a contained unit that produces electricity, whereas a fuel cell. Fuel Cells In Acidic Electrolyte.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They are typically used in. Fuel Cells In Acidic Electrolyte.
From www.slideserve.com
PPT Phosphoric Acid Fuel cell PowerPoint Presentation, free download Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. They are typically used in modules of 400 kw or. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel. Fuel Cells In Acidic Electrolyte.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They are typically used in modules. Fuel Cells In Acidic Electrolyte.
From blogs.rsc.org
HOT Articles Page 2 Environmental Science Water Research Fuel Cells In Acidic Electrolyte Fuel cells have an important advantage over all. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They are typically used in modules of 400 kw or. This review discusses the history, fundamentals, and applications of different fuel. Fuel Cells In Acidic Electrolyte.
From dailyenergyinsider.com
DOE awards additional 8M to FuelCell Energy in pursuit of solid oxide Fuel Cells In Acidic Electrolyte They are typically used in modules of 400 kw or. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Fuel cells have an important advantage over all. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A battery is a. Fuel Cells In Acidic Electrolyte.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage over all. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. This review discusses the history,. Fuel Cells In Acidic Electrolyte.
From saylordotorg.github.io
Electrochemistry Fuel Cells In Acidic Electrolyte This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. Fuel cells have an important advantage over all. A fuel cell like this will continue to operate and produce electrical energy. Fuel Cells In Acidic Electrolyte.
From www.researchgate.net
Polymerelectrolytefuelcell schematic showing the reactantgas and Fuel Cells In Acidic Electrolyte Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They are typically used in modules of 400 kw or. Fuel cells have an. Fuel Cells In Acidic Electrolyte.
From www.mdpi.com
Polymers Free FullText Water Soluble Polymers as Proton Exchange Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A battery is a contained unit that produces electricity, whereas a fuel cell is a. Fuel Cells In Acidic Electrolyte.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use. Fuel Cells In Acidic Electrolyte.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Circuit Diagram For Electrolysis Of Water Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about. Fuel Cells In Acidic Electrolyte.
From exyueugsn.blob.core.windows.net
Do All Fuel Cells Use Hydrogen at Donald Venable blog Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held. Fuel Cells In Acidic Electrolyte.
From www.prweb.com
NEI Corporation Extends Its Capabilities to Develop PEMs for Fuel Cells Fuel Cells In Acidic Electrolyte A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held. Fuel Cells In Acidic Electrolyte.
From www.energy.gov
Hydrogen Production Electrolysis Department of Energy Fuel Cells In Acidic Electrolyte The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. This review discusses the history, fundamentals,. Fuel Cells In Acidic Electrolyte.
From www.vedantu.com
Draw a neat labelled diagram of {H_2} {O_2} fuel cell. Write the Fuel Cells In Acidic Electrolyte This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. The phosphoric acid fuel cell (pafc) includes the usage of acid electrolytes, which also allows the passage of positively charged h + ions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and. Fuel Cells In Acidic Electrolyte.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cells In Acidic Electrolyte Fuel cells have an important advantage over all. They are typically used in modules of 400 kw or. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that. Fuel Cells In Acidic Electrolyte.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Fuel Cells In Acidic Electrolyte They are typically used in modules of 400 kw or. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. The phosphoric acid fuel. Fuel Cells In Acidic Electrolyte.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cells In Acidic Electrolyte This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. A fuel cell like this will continue to operate and produce electrical. Fuel Cells In Acidic Electrolyte.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Fuel Cells In Acidic Electrolyte This review discusses the history, fundamentals, and applications of different fuel cell technologies, including proton exchange. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at. Fuel Cells In Acidic Electrolyte.
From web.stanford.edu
PEM Fuel Cells Fuel Cells In Acidic Electrolyte A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They are typically used in modules of 400 kw or. Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°c. Fuel cells have an. Fuel Cells In Acidic Electrolyte.