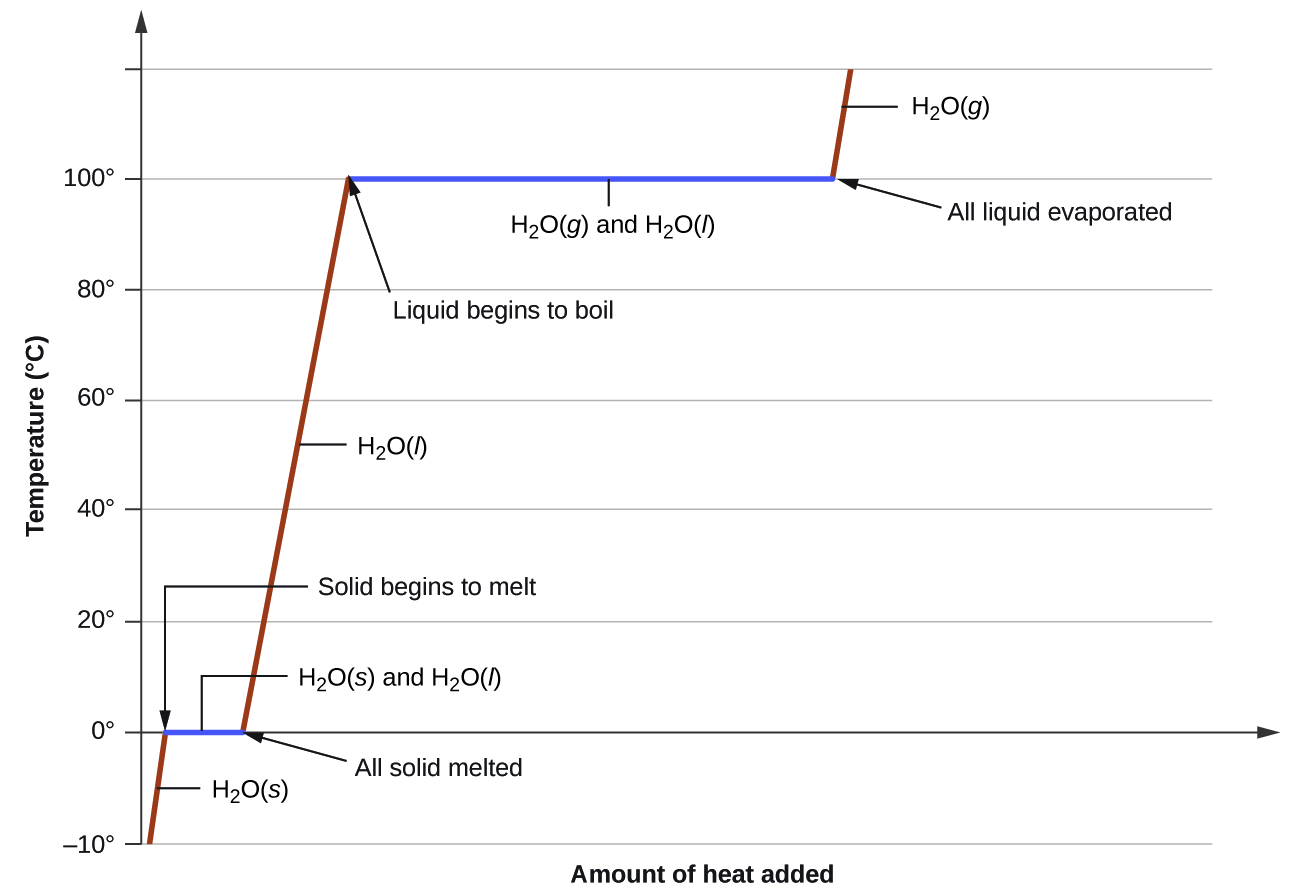
Heating Curve For Acetone . C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. The original sources often give temperature ranges over which these constants should be used. C 3 h 6 o. However, we can normally extrapolate beyond. Plateaus in the curve (regions of constant temperature) are. Like specific heat capacities, heats of fusion vary from one substance to another. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Note that water has a relatively high. It also shows the saturation pressure with changes in temperature. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm.
from ar.inspiredpencil.com
C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. However, we can normally extrapolate beyond. Note that water has a relatively high. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. It also shows the saturation pressure with changes in temperature. Like specific heat capacities, heats of fusion vary from one substance to another. C 3 h 6 o. The original sources often give temperature ranges over which these constants should be used. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure.
Heating Curves In Chemistry
Heating Curve For Acetone However, we can normally extrapolate beyond. C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. Like specific heat capacities, heats of fusion vary from one substance to another. The original sources often give temperature ranges over which these constants should be used. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Note that water has a relatively high. C 3 h 6 o. However, we can normally extrapolate beyond. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. It also shows the saturation pressure with changes in temperature.
From lewiskainui.blogspot.com
11+ Heating Curve Calculations LewisKainui Heating Curve For Acetone Like specific heat capacities, heats of fusion vary from one substance to another. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. C 3 h 6 o. It also shows the saturation pressure. Heating Curve For Acetone.
From answerlistclapnets.z14.web.core.windows.net
Calculation Of Heating Curve For Water Heating Curve For Acetone A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. C 3 h 6 o. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. The original sources often give temperature ranges over which these constants should be. Heating Curve For Acetone.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Heating Curve For Acetone C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. Like specific heat capacities, heats of fusion vary from one substance to another. The original sources often give temperature ranges over which these constants should be used. Note that water has a relatively high. A) a heating curve. Heating Curve For Acetone.
From joirurhka.blob.core.windows.net
Temperature Change Experiment at Deanna Bickford blog Heating Curve For Acetone However, we can normally extrapolate beyond. Plateaus in the curve (regions of constant temperature) are. C 3 h 6 o. C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. Note that water has a relatively high. It also shows the saturation pressure with changes in temperature. The. Heating Curve For Acetone.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Curve For Acetone Like specific heat capacities, heats of fusion vary from one substance to another. C 3 h 6 o. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Plateaus in the curve (regions of constant temperature) are. Note that water has a relatively high. A) a heating curve for water depicts. Heating Curve For Acetone.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve For Acetone C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Like specific heat capacities, heats of fusion vary from one substance to another. The original sources often give temperature ranges over which these constants should be used.. Heating Curve For Acetone.
From lessonfullantje.z19.web.core.windows.net
Draw And Label The Heating Curve For Water Heating Curve For Acetone However, we can normally extrapolate beyond. Note that water has a relatively high. C 3 h 6 o. The original sources often give temperature ranges over which these constants should be used. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. The curve between the critical point and the triple point shows. Heating Curve For Acetone.
From www.researchgate.net
a) The inner working circuit of the home‐made sensor and the heating Heating Curve For Acetone Note that water has a relatively high. It also shows the saturation pressure with changes in temperature. Plateaus in the curve (regions of constant temperature) are. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. C 3 h 6 o. The original sources often give temperature ranges over which these. Heating Curve For Acetone.
From slideplayer.com
Chapter 11 intermolecular forces (IMF) attractive forces between all Heating Curve For Acetone Note that water has a relatively high. Like specific heat capacities, heats of fusion vary from one substance to another. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm.. Heating Curve For Acetone.
From preparatorychemistry.com
Heating Curve Heating Curve For Acetone For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. The original sources often give temperature ranges over which these constants should be used. C 3 h 6 o. However, we can normally extrapolate beyond. C 3 h 6 o. A) a heating curve for water depicts changes in temperature that result as. Heating Curve For Acetone.
From ar.inspiredpencil.com
Heating Curves In Chemistry Heating Curve For Acetone However, we can normally extrapolate beyond. The original sources often give temperature ranges over which these constants should be used. Note that water has a relatively high. It also shows the saturation pressure with changes in temperature. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm.. Heating Curve For Acetone.
From lessonlibnurselings.z21.web.core.windows.net
Heating Curve Of Water Explained Heating Curve For Acetone A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. It also shows the saturation pressure with changes in temperature. C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. Note that water has a. Heating Curve For Acetone.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation ID6558754 Heating Curve For Acetone C 3 h 6 o. Note that water has a relatively high. However, we can normally extrapolate beyond. The original sources often give temperature ranges over which these constants should be used. It also shows the saturation pressure with changes in temperature. C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and. Heating Curve For Acetone.
From www.researchgate.net
DSC results a heatcoolheat curve for HPMC5 wt CA sample; b first Heating Curve For Acetone C 3 h 6 o. Like specific heat capacities, heats of fusion vary from one substance to another. Plateaus in the curve (regions of constant temperature) are. It also shows the saturation pressure with changes in temperature. The original sources often give temperature ranges over which these constants should be used. A) a heating curve for water depicts changes in. Heating Curve For Acetone.
From www.youtube.com
How to Read a Heating Curve YouTube Heating Curve For Acetone The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. The original sources often give temperature ranges over which these constants should be used. C 3 h 6 o. Like specific heat capacities, heats of fusion vary. Heating Curve For Acetone.
From www.liveworksheets.com
Heating curve 0552 kh Live Worksheets Heating Curve For Acetone C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. C 3 h 6 o. Note that water has a relatively high. The original sources often give temperature ranges over which these constants should be used. A) a heating curve for water depicts changes in temperature that result. Heating Curve For Acetone.
From flexbooks.ck12.org
CK12Foundation Heating Curve For Acetone C 3 h 6 o. The original sources often give temperature ranges over which these constants should be used. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Like specific heat capacities, heats of fusion vary from one substance to another. It also shows the saturation pressure with changes in. Heating Curve For Acetone.
From askfilo.com
The graph shows the heating curve for a pure substance. The temperature r.. Heating Curve For Acetone Note that water has a relatively high. Plateaus in the curve (regions of constant temperature) are. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. C 3 h 6. Heating Curve For Acetone.
From www.numerade.com
SOLVED a) What does heating curve show? b) What does cooling curve Heating Curve For Acetone It also shows the saturation pressure with changes in temperature. The original sources often give temperature ranges over which these constants should be used. However, we can normally extrapolate beyond. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. Note that water has a relatively high. Like specific heat capacities, heats of. Heating Curve For Acetone.
From markusghopchung.blogspot.com
Critical Temperature of Methanol Heating Curve For Acetone Plateaus in the curve (regions of constant temperature) are. C 3 h 6 o. C 3 h 6 o. Note that water has a relatively high. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. However, we can normally extrapolate beyond. It also shows the saturation pressure with changes in temperature. The. Heating Curve For Acetone.
From www.studocu.com
Heating curve wrksht v2 Heating Curve Worksheet Name period Date Heating Curve For Acetone Like specific heat capacities, heats of fusion vary from one substance to another. The original sources often give temperature ranges over which these constants should be used. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. C 3 h 6 o. A) a heating curve for water depicts changes in. Heating Curve For Acetone.
From www.chegg.com
Solved The vapor pressure curves for acetone, ethanol and Heating Curve For Acetone Like specific heat capacities, heats of fusion vary from one substance to another. The original sources often give temperature ranges over which these constants should be used. C 3 h 6 o. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. It also shows the saturation pressure with changes in. Heating Curve For Acetone.
From www.numerade.com
SOLVED 016 Heating curve multipart calculation 9 Points Use the table Heating Curve For Acetone C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. The original sources often give temperature ranges over which these constants should be used. However,. Heating Curve For Acetone.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve For Acetone A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. It also shows the saturation pressure with changes in temperature. Note that water has a relatively high. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. C 3 h. Heating Curve For Acetone.
From ar.inspiredpencil.com
Heating Curve Diagram Heating Curve For Acetone Note that water has a relatively high. It also shows the saturation pressure with changes in temperature. C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. Like specific heat capacities, heats of fusion vary from one substance to another. A) a heating curve for water depicts changes in temperature that result as the substance absorbs. Heating Curve For Acetone.
From curiophysics.com
Heating Curve » Curio Physics Heating Curve For Acetone Like specific heat capacities, heats of fusion vary from one substance to another. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Note that water has a relatively high. C 3 h 6 o. However, we can normally extrapolate beyond. C 3 h 6 o. The original sources often give. Heating Curve For Acetone.
From wordwall.net
Heating curve Labelled diagram Heating Curve For Acetone For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Like specific heat capacities, heats of fusion vary from one substance to another. C 3 h 6 o. However, we. Heating Curve For Acetone.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Curve For Acetone A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. C 3 h 6 o. C 3 h 6 o. It also shows the saturation pressure with changes in temperature. However, we can normally extrapolate beyond. Plateaus in the curve (regions of constant temperature) are. Like specific. Heating Curve For Acetone.
From www.npro.energy
Heating curve for building energy systems nPro Heating Curve For Acetone Plateaus in the curve (regions of constant temperature) are. The original sources often give temperature ranges over which these constants should be used. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Note that water has a relatively high. C 3 h 6 o. Like specific heat capacities, heats of. Heating Curve For Acetone.
From mavink.com
Acetone Water Phase Diagram Heating Curve For Acetone The original sources often give temperature ranges over which these constants should be used. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. However, we can normally extrapolate beyond. C 3 h 6 o. Note that water has a relatively high. Plateaus in the curve (regions of constant temperature) are. A) a. Heating Curve For Acetone.
From wordwall.net
Heating curve 2 Labelled diagram Heating Curve For Acetone The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Plateaus in the curve (regions of constant temperature) are. It also shows the saturation pressure with changes in temperature. Note that water has a relatively high. C 3 h 6 o. A) a heating curve for water depicts changes in temperature. Heating Curve For Acetone.
From www.numerade.com
Heating Curve for Methanol J00 450 A The graph above represents the Heating Curve For Acetone The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. C 3 h 6 o. For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. It also shows the saturation pressure with changes in temperature. Like specific heat capacities, heats of fusion vary from. Heating Curve For Acetone.
From www.numerade.com
SOLVED Use the heating curve for acetic acid to answer the following Heating Curve For Acetone For example, it is 333.55 j/g for water, 97.99 j/g for acetone, and 58.99 j/g for methane. C 3 h 6 o. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. C 3 h 6 o. It also shows the saturation pressure with changes in temperature.. Heating Curve For Acetone.
From solvedlib.com
Draw a heating curve for a sample of ice that is heat… SolvedLib Heating Curve For Acetone The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. The original sources often give temperature ranges over which these constants should be used. Plateaus in the curve (regions of constant temperature) are. Note that water has a relatively high. For example, it is 333.55 j/g for water, 97.99 j/g for. Heating Curve For Acetone.
From www.slideserve.com
PPT Behavior of Gases PowerPoint Presentation, free download ID6558754 Heating Curve For Acetone The original sources often give temperature ranges over which these constants should be used. A) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. C 3 h 6 o. Plateaus in the curve (regions of constant temperature) are. For example, it is 333.55 j/g for water, 97.99. Heating Curve For Acetone.