Virtual Lab Flame Test And Spectroscopy . Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. 1.observe the bright line spectra (emission spectra) for various elements. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. On my teacher website, find the link for the spectroscopy virtual lab (part 1: The observed spectrum looks like a. In this virtual lab you will: Flame tests and identification of an unknown metal) to discover. 2.use a flame test to observe the color. When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Then using a spectroscope, match the bright line spectra. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Use a flame test to determine which ion salt produces the red color.
from studylib.net
Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Use a flame test to determine which ion salt produces the red color. 1.observe the bright line spectra (emission spectra) for various elements. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Flame tests and identification of an unknown metal) to discover. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. The observed spectrum looks like a. 2.use a flame test to observe the color. In this virtual lab you will: Then using a spectroscope, match the bright line spectra.
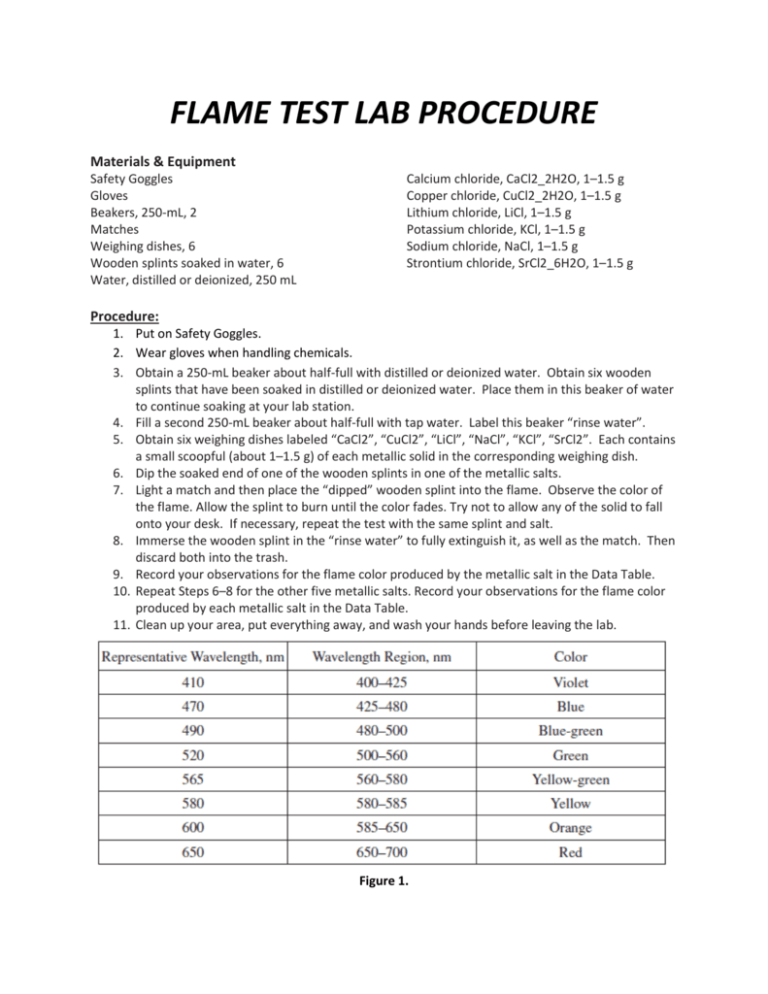
FLAME TEST LAB PROCEDURE
Virtual Lab Flame Test And Spectroscopy Use a flame test to determine which ion salt produces the red color. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. 2.use a flame test to observe the color. In this virtual lab you will: The observed spectrum looks like a. Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line spectra. Flame tests and identification of an unknown metal) to discover. 1.observe the bright line spectra (emission spectra) for various elements. On my teacher website, find the link for the spectroscopy virtual lab (part 1: When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear.
From www.scribd.com
Flame Test & Spectroscopy Virtual Lab PDF Emission Spectrum Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Then using a spectroscope, match the bright line spectra. Founded in 2002 by nobel laureate carl wieman, the phet. Virtual Lab Flame Test And Spectroscopy.
From quizizz.com
Quizizz Virtual Lab Flame Test And Spectroscopy The observed spectrum looks like a. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. 1.observe the bright line spectra (emission spectra) for various elements. When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light. Virtual Lab Flame Test And Spectroscopy.
From www.scribd.com
Flame Test Lab Example Emission Spectrum Atoms Virtual Lab Flame Test And Spectroscopy Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Use a flame test to determine which ion salt produces the red. Virtual Lab Flame Test And Spectroscopy.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Virtual Lab Flame Test And Spectroscopy The observed spectrum looks like a. 1.observe the bright line spectra (emission spectra) for various elements. Use a flame test to determine which ion salt produces the red color. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Learn flame test procedure and how to detect the presence. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
baking soda Mr. Palermo's Flipped Chemistry Classroom Virtual Lab Flame Test And Spectroscopy The observed spectrum looks like a. 1.observe the bright line spectra (emission spectra) for various elements. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Then using a spectroscope, match the bright line spectra. Flame tests and identification of an unknown metal) to discover. Use a flame test to. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
How To Flame Test Lab 🔥 YouTube Virtual Lab Flame Test And Spectroscopy When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Then using a spectroscope, match the bright line spectra. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. The observed spectrum looks like. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
lemon juice Mr. Palermo's Flipped Chemistry Classroom Virtual Lab Flame Test And Spectroscopy When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. 2.use a flame test to observe the color. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Founded in 2002 by nobel laureate carl. Virtual Lab Flame Test And Spectroscopy.
From scienceready.com.au
Atomic Emission Spectroscopy & Flame Test HSC Chemistry Science Ready Virtual Lab Flame Test And Spectroscopy Then using a spectroscope, match the bright line spectra. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Flame tests and identification of an unknown metal) to discover. 1.observe the bright line spectra (emission spectra) for various elements. Use a flame test to determine which ion salt produces. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Flame Test Lab YouTube Virtual Lab Flame Test And Spectroscopy Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. On my teacher website, find the link for the spectroscopy virtual lab (part 1: The observed spectrum looks like a. 2.use a flame test to observe the color. 1.observe the bright line spectra. Virtual Lab Flame Test And Spectroscopy.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Virtual Lab Flame Test And Spectroscopy Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project. Virtual Lab Flame Test And Spectroscopy.
From ceuhgllz.blob.core.windows.net
Flame Atomic Absorption Spectrometry Traduccion at Heather Clement blog Virtual Lab Flame Test And Spectroscopy 2.use a flame test to observe the color. Then using a spectroscope, match the bright line spectra. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. In this virtual lab you will: The observed spectrum looks like a. 1.observe the bright line spectra (emission spectra) for various elements.. Virtual Lab Flame Test And Spectroscopy.
From www.slideserve.com
PPT Flame Spectroscopy Lab PowerPoint Presentation, free download Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: 2.use a flame test to observe the color. Use a flame test to determine which ion salt produces the red color. The observed spectrum looks like a. In this virtual lab you will: Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project. Virtual Lab Flame Test And Spectroscopy.
From www.blendspace.com
Flame Test Virtual Lab Lessons Blendspace Virtual Lab Flame Test And Spectroscopy The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. 1.observe the bright line spectra (emission spectra) for various elements. 2.use a flame test to observe the color. Learn. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Measuring flame temperature with thermocouple virtual laboratory Virtual Lab Flame Test And Spectroscopy Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. 1.observe the bright line spectra (emission spectra) for. Virtual Lab Flame Test And Spectroscopy.
From www.studocu.com
Copy of Flame Test Virtual Lab Flame Test Virtual Lab Document Virtual Lab Flame Test And Spectroscopy Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Flame tests and identification of an unknown metal) to discover. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. The observed. Virtual Lab Flame Test And Spectroscopy.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line spectra. Flame tests and identification of an unknown metal) to discover. Learn flame test procedure and how to detect the presence of metal ions in. Virtual Lab Flame Test And Spectroscopy.
From www.alamyimages.fr
test de flamme. la procédure de chimie analytique a utilisé la couleur Virtual Lab Flame Test And Spectroscopy In this virtual lab you will: Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. Flame tests and identification of an unknown metal) to discover. Then using a spectroscope, match the bright line spectra. Use a flame test to determine which ion. Virtual Lab Flame Test And Spectroscopy.
From db-excel.com
Flame Test Lab Worksheet Answer Key — Virtual Lab Flame Test And Spectroscopy The observed spectrum looks like a. In this virtual lab you will: 1.observe the bright line spectra (emission spectra) for various elements. Flame tests and identification of an unknown metal) to discover. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. Founded in 2002 by nobel laureate carl wieman,. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Atomic Spectroscopy Flame Emission The Flame Test YouTube Virtual Lab Flame Test And Spectroscopy Then using a spectroscope, match the bright line spectra. 2.use a flame test to observe the color. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test virtual lab. In this virtual lab you will: On my teacher website, find the link for the spectroscopy. Virtual Lab Flame Test And Spectroscopy.
From studylib.net
FLAME TEST LAB PROCEDURE Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. In this virtual lab you will: The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic. Virtual Lab Flame Test And Spectroscopy.
From brokeasshome.com
Glencoe Virtual Lab Periodic Table Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. The observed spectrum looks like a. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form. Virtual Lab Flame Test And Spectroscopy.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line spectra. The observed spectrum looks like a. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado. Virtual Lab Flame Test And Spectroscopy.
From kbizdesign.blogspot.com
Explain How Colors In The Flame Test Are Produced Virtual Lab Flame Test And Spectroscopy 1.observe the bright line spectra (emission spectra) for various elements. The observed spectrum looks like a. Then using a spectroscope, match the bright line spectra. Use a flame test to determine which ion salt produces the red color. On my teacher website, find the link for the spectroscopy virtual lab (part 1: Learn flame test procedure and how to detect. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Virtual Lab Flame Test And Spectroscopy 2.use a flame test to observe the color. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. In this virtual lab you will: On my teacher website, find the link for the spectroscopy virtual lab (part 1: The observed spectrum looks like a. Use a flame test to determine. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
apple juice Mr. Palermo's Flipped Chemistry Classroom Virtual Lab Flame Test And Spectroscopy On my teacher website, find the link for the spectroscopy virtual lab (part 1: When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions. Virtual Lab Flame Test And Spectroscopy.
From knowledge.carolina.com
Flame Tests and Spectroscopy Get Excited About Color Carolina Virtual Lab Flame Test And Spectroscopy The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. 1.observe the bright line spectra (emission spectra) for various elements. When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Flame tests and identification of. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Virtual Lab Flame Test And Spectroscopy 2.use a flame test to observe the color. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Then using a spectroscope, match the bright line spectra. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in. Virtual Lab Flame Test And Spectroscopy.
From learningricardo.z19.web.core.windows.net
Flame Test Lab Worksheet Answer Key Chemistry Virtual Lab Flame Test And Spectroscopy 2.use a flame test to observe the color. In this virtual lab you will: Use a flame test to determine which ion salt produces the red color. Then using a spectroscope, match the bright line spectra. Flame tests and identification of an unknown metal) to discover. Learn flame test procedure and how to detect the presence of metal ions in. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Virtual Lab Flame Test And Spectroscopy Then using a spectroscope, match the bright line spectra. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. 1.observe the bright line spectra (emission spectra) for various elements. Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free.. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Flame Atomic Absorption Spectroscopy Demonstration YouTube Virtual Lab Flame Test And Spectroscopy When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Use a flame test to determine which ion salt produces the red color. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. On my. Virtual Lab Flame Test And Spectroscopy.
From www.mrpalermo.com
shampoo Mr. Palermo's Flipped Chemistry Classroom Virtual Lab Flame Test And Spectroscopy Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. Then using a spectroscope, match the bright line spectra. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and identify the characteristic colors. On my teacher website, find the link for the spectroscopy. Virtual Lab Flame Test And Spectroscopy.
From www.youtube.com
Flame Test Lab YouTube Virtual Lab Flame Test And Spectroscopy Flame tests and identification of an unknown metal) to discover. In this virtual lab you will: Use a flame test to determine which ion salt produces the red color. On my teacher website, find the link for the spectroscopy virtual lab (part 1: 1.observe the bright line spectra (emission spectra) for various elements. Learn flame test procedure and how to. Virtual Lab Flame Test And Spectroscopy.
From www.formsbank.com
Flame Test Lab printable pdf download Virtual Lab Flame Test And Spectroscopy In this virtual lab you will: Then using a spectroscope, match the bright line spectra. 2.use a flame test to observe the color. 1.observe the bright line spectra (emission spectra) for various elements. Use a flame test to determine which ion salt produces the red color. Learn flame test procedure and how to detect the presence of metal ions in. Virtual Lab Flame Test And Spectroscopy.
From www.animalia-life.club
Flame Test Lab Results Virtual Lab Flame Test And Spectroscopy Founded in 2002 by nobel laureate carl wieman, the phet interactive simulations project at the university of colorado boulder creates free. On my teacher website, find the link for the spectroscopy virtual lab (part 1: Then using a spectroscope, match the bright line spectra. The flame test virtual lab is a simulated laboratory experiment that allows students to observe and. Virtual Lab Flame Test And Spectroscopy.
From www.pinterest.com
Pin on Technology Virtual Lab Flame Test And Spectroscopy 1.observe the bright line spectra (emission spectra) for various elements. When looking at the spectrum of light emitted from a fluorescent lamp, sodium lamp, neon sign, or flame test, only distinct wavelengths of light appear. Learn flame test procedure and how to detect the presence of metal ions in their salts either, powder form or solutions in the flame test. Virtual Lab Flame Test And Spectroscopy.