Osmolarity Equation Chemistry . Number of particles that dissociated from the solute molecule. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. Osmolarity is nearly the same as. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. An osmole is 1 mol of particles that. osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. osmolality is a measure of the concentration of particles in the serum per kilogram of water.
from www.chegg.com
osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Osmolarity is nearly the same as. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity = molarity x n x f. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of osmoles of solute per litre of solution. Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that.
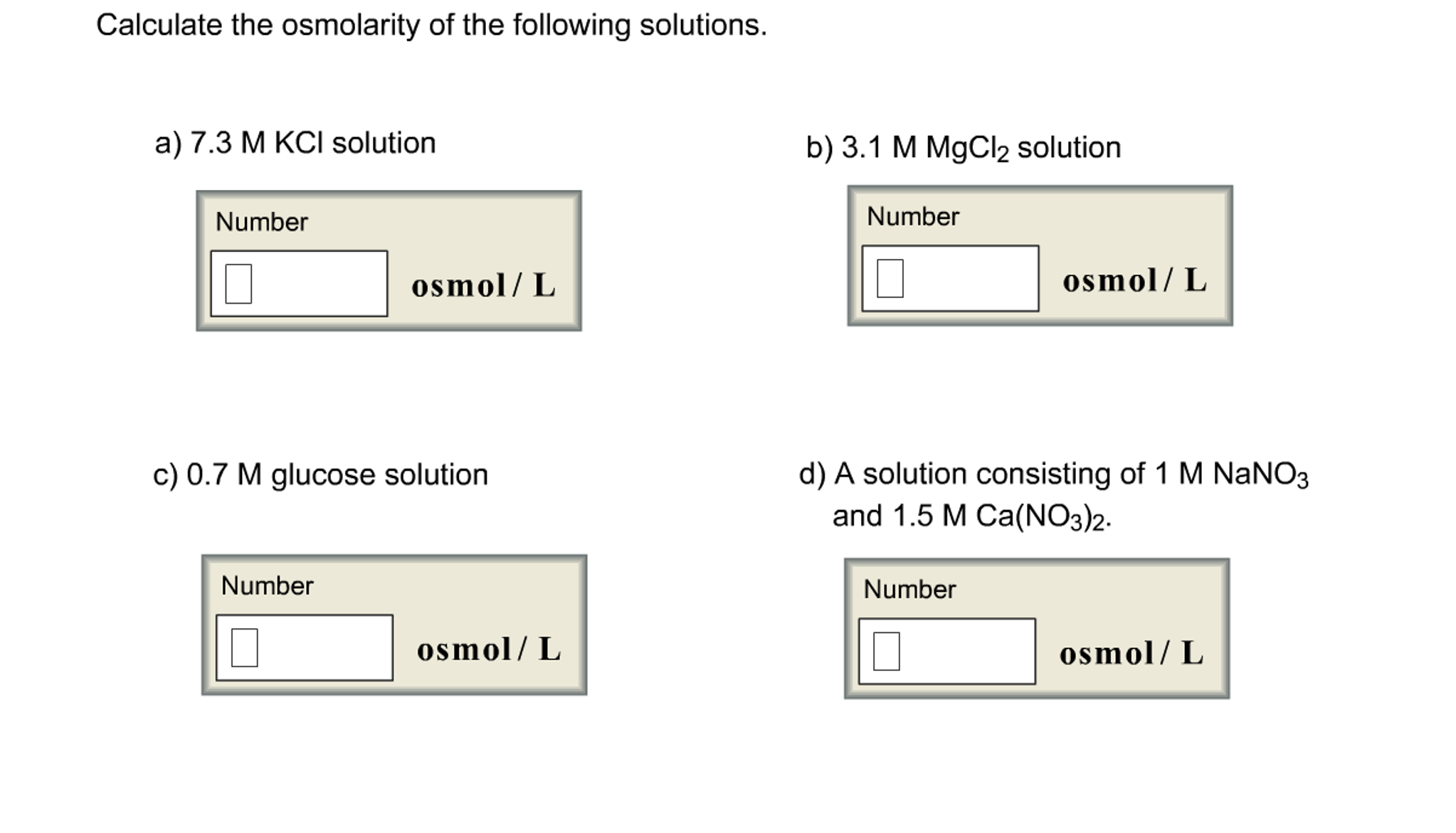
Solved Calculate the osmolarity of the following solutions.
Osmolarity Equation Chemistry osmolarity = molarity x n x f. Number of particles that dissociated from the solute molecule. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. An osmole is 1 mol of particles that. osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolarity = molarity x n x f.
From www.numerade.com
SOLVED A typical range of osmolarity of cells and extracellular fluid Osmolarity Equation Chemistry osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that. osmolarity = molarity x n x f. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution. Osmolarity Equation Chemistry.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube Osmolarity Equation Chemistry osmolality is a measure of the concentration of particles in the serum per kilogram of water. Osmolarity is nearly the same as. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. osmolarity = molarity. Osmolarity Equation Chemistry.
From www.youtube.com
Properties of Solutions 18 Osmolality, Osmolarity, and van't Hoff Osmolarity Equation Chemistry Number of particles that dissociated from the solute molecule. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Osmolarity. Osmolarity Equation Chemistry.
From www.slideserve.com
PPT Osmolar Gaps How does EtOH contribute to osmolar gaps? Can Osmolarity Equation Chemistry osmolality is a measure of the concentration of particles in the serum per kilogram of water. Number of particles that dissociated from the solute molecule. Osmolarity is nearly the same as. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or liter) of. Osmolarity Equation Chemistry.
From askfilo.com
The osmolarity of the glomerular filtrate at the bottom of loop of Henle'.. Osmolarity Equation Chemistry Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. Osmolarity is nearly the same as.. Osmolarity Equation Chemistry.
From www.slideshare.net
Membrane Dynamics1 Osmolarity Equation Chemistry osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity = molarity x n x f. Osmolarity is nearly the same as. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. An osmole is 1 mol of particles that. . Osmolarity Equation Chemistry.
From www.researchgate.net
Composition and osmolarity (Osm/L) of DSW and NaCl solutions. Figures Osmolarity Equation Chemistry Osmolarity is nearly the same as. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of osmoles of solute per litre of solution. osmolarity = molarity x n x f. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution.. Osmolarity Equation Chemistry.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube Osmolarity Equation Chemistry osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity = molarity x n x. Osmolarity Equation Chemistry.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmolarity Equation Chemistry An osmole is 1 mol of particles that. Osmolarity is nearly the same as. Number of particles that dissociated from the solute molecule. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity (osmol) is a way of reporting the total number of particles in a solution to. Osmolarity Equation Chemistry.
From ibiologia.com
How to calculate Osmolarity from Molarity? Osmolarity Equation Chemistry osmolarity = molarity x n x f. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of osmoles of solute per litre of solution. Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that. osmolality is milliosmoles of. Osmolarity Equation Chemistry.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolarity Equation Chemistry Number of particles that dissociated from the solute molecule. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity = molarity x n x f. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of. Osmolarity Equation Chemistry.
From www.youtube.com
Calculated Osmolality YouTube Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. Osmolarity is nearly the same as. osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol. Osmolarity Equation Chemistry.
From www.youtube.com
Estimating Serum Osmolality using a Simple Formula YouTube Osmolarity Equation Chemistry osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated. Osmolarity Equation Chemistry.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. Osmolarity is nearly the same as. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity (osmol) is. Osmolarity Equation Chemistry.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. An osmole is 1 mol of particles that. osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number. Osmolarity Equation Chemistry.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. An osmole is 1 mol of particles that. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity (osmol) is a way of reporting the total number of particles in a. Osmolarity Equation Chemistry.
From study.com
Osmolarity Definition, Formula & Calculations Video & Lesson Osmolarity Equation Chemistry An osmole is 1 mol of particles that. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity. Osmolarity Equation Chemistry.
From www.youtube.com
How to solve osmolarity calculation problems YouTube Osmolarity Equation Chemistry osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. Number of particles that dissociated from the solute molecule. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is a measure of the concentration of particles in the serum per kilogram. Osmolarity Equation Chemistry.
From questions.kunduz.com
16. Show the calculation of osmolarity for... Organic Chemistry Osmolarity Equation Chemistry osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. Number of particles that dissociated from. Osmolarity Equation Chemistry.
From www.slideserve.com
PPT Chemical calculations used in medicine part 1 PowerPoint Osmolarity Equation Chemistry osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolarity is the number of osmoles of solute per litre of solution. Osmolarity is nearly the same as. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is a measure. Osmolarity Equation Chemistry.
From www.youtube.com
Tonicity & Osmolarity YouTube Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. An osmole is 1 mol of particles that. osmolality is a measure of the concentration of particles in the serum per kilogram of water. Number of particles that dissociated from the solute molecule. osmolarity = molarity x n. Osmolarity Equation Chemistry.
From www.pinterest.com
the word osmoliaity is written in two different languages on a blue Osmolarity Equation Chemistry osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. Number of particles that dissociated from the solute molecule. osmolarity (osmol) is a way of reporting the total number of particles in. Osmolarity Equation Chemistry.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube Osmolarity Equation Chemistry osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity is the number of osmoles. Osmolarity Equation Chemistry.
From www.showme.com
Osmolarity of a dose 1 Math ShowMe Osmolarity Equation Chemistry osmolality is a measure of the concentration of particles in the serum per kilogram of water. Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or. Osmolarity Equation Chemistry.
From www.slideserve.com
PPT Osmolar Gaps How does EtOH contribute to osmolar gaps? Can Osmolarity Equation Chemistry Osmolarity is nearly the same as. osmolarity = molarity x n x f. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolarity is defined as the number of milliosmoles of the solutes per. Osmolarity Equation Chemistry.
From drawittoknowit.com
Anatomy & Physiology Osmosis and Osmolarity ditki medical Osmolarity Equation Chemistry osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolarity = molarity x n x f. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma). Osmolarity Equation Chemistry.
From www.bartleby.com
Answered Determine the osmolarity of 7.0 × 103 M… bartleby Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. Osmolarity is nearly the same as. Number of particles that dissociated from the solute molecule. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolarity = molarity x. Osmolarity Equation Chemistry.
From study.com
Serum Osmolality Definition, Calculation & Interpretation Lesson Osmolarity Equation Chemistry osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. Number of particles that dissociated from the solute molecule. Osmolarity is nearly the same as. osmolarity is defined as the number of milliosmoles of the solutes. Osmolarity Equation Chemistry.
From www.youtube.com
Osmolarity Example (Bio) YouTube Osmolarity Equation Chemistry An osmole is 1 mol of particles that. osmolality is a measure of the concentration of particles in the serum per kilogram of water. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution. Osmolarity Equation Chemistry.
From www.coursehero.com
[Solved] Calculate osmolarity Li3N 1g 500ml Course Hero Osmolarity Equation Chemistry osmolarity = molarity x n x f. Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity (osmol) is a way of reporting the total number of particles in a. Osmolarity Equation Chemistry.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmolarity Equation Chemistry osmolarity = molarity x n x f. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. Osmolarity is nearly the same as. osmolality is a measure of the concentration of particles in the serum per kilogram of water. Number of particles that dissociated from the solute molecule.. Osmolarity Equation Chemistry.
From www.pinterest.com
Vant Hoff formula For calculation of Osmotic pressure ( Note Osmolarity Equation Chemistry Number of particles that dissociated from the solute molecule. An osmole is 1 mol of particles that. Osmolarity is nearly the same as. osmolarity is the number of osmoles of solute per litre of solution. osmolarity (osmol) is a way of reporting the total number of particles in a solution to determine osmotic pressure. osmolality is a. Osmolarity Equation Chemistry.
From www.chegg.com
Solved Calculate the osmolarity of the following solutions. Osmolarity Equation Chemistry osmolarity = molarity x n x f. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. osmolality is milliosmoles of solutes per one kilogram (or liter) of water of solution (plasma) and is calculated by. osmolarity is the number of osmoles of solute per litre of solution. An osmole is. Osmolarity Equation Chemistry.
From dxoxftzse.blob.core.windows.net
How To Calculate Osmolarity Of D5W at Marin Wyatt blog Osmolarity Equation Chemistry osmolarity is the number of osmoles of solute per litre of solution. Number of particles that dissociated from the solute molecule. osmolality is a measure of the concentration of particles in the serum per kilogram of water. An osmole is 1 mol of particles that. osmolarity (osmol) is a way of reporting the total number of particles. Osmolarity Equation Chemistry.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical Osmolarity Equation Chemistry Osmolarity is nearly the same as. osmolarity is defined as the number of milliosmoles of the solutes per liter of solution. An osmole is 1 mol of particles that. Number of particles that dissociated from the solute molecule. osmolarity = molarity x n x f. osmolality is a measure of the concentration of particles in the serum. Osmolarity Equation Chemistry.