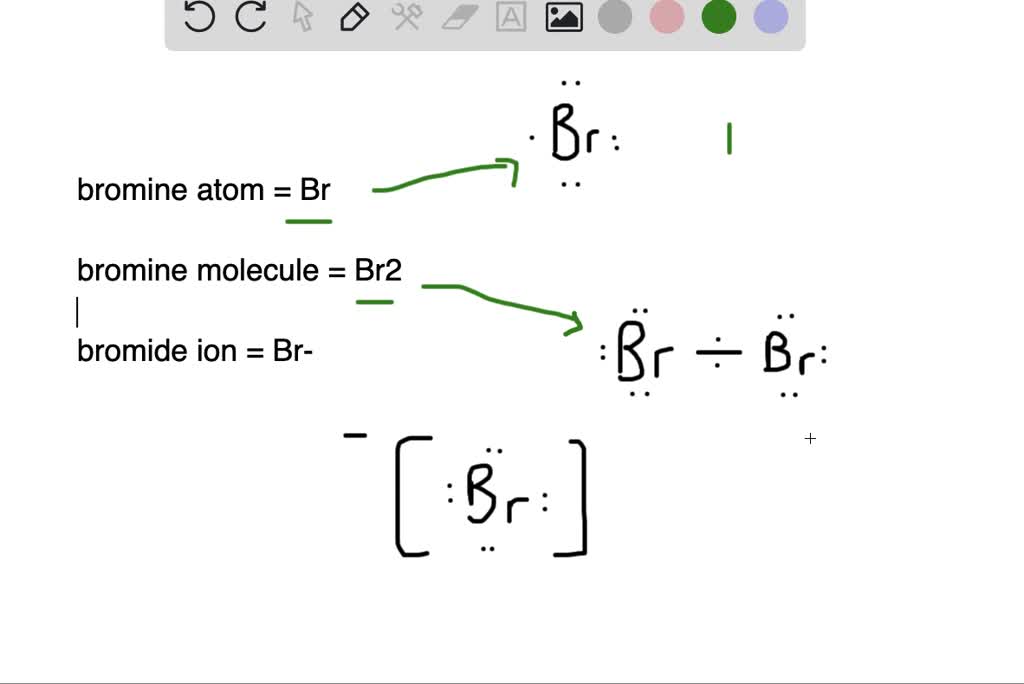
Compounds Bromine Form . Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Natural salt deposits and brines are the main sources of. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature. They form polar covalent bonds in. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr).
from dxofeotiz.blob.core.windows.net
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature. They form polar covalent bonds in. Natural salt deposits and brines are the main sources of.
Bromine A Compound at Dawn Bocanegra blog
Compounds Bromine Form They form polar covalent bonds in. They form polar covalent bonds in. Natural salt deposits and brines are the main sources of. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr).
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Natural salt deposits and brines are the main sources of. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on. Compounds Bromine Form.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Natural salt deposits and brines are the main sources of. Bromine atoms are highly electronegative, with an electronegativity. Compounds Bromine Form.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Compounds Bromine Form Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine is too reactive to exist as a free element in nature. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Natural salt deposits and brines are the main sources of. Bromine, a halogen element with. Compounds Bromine Form.
From answerhappy.com
During any aromatic bromination, both compounds shown will form a Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as. Compounds Bromine Form.
From www.numerade.com
SOLVED Which pairs of elements will form ionic compounds? cesium and Compounds Bromine Form This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. They form polar covalent bonds in. Natural salt deposits and brines are the main sources of. Instead, it occurs in compounds, the most common of which are sodium bromide. Compounds Bromine Form.
From www.vedantu.com
Reaction of bromine water phenol gives white ppt ofA. o bromophenolB Compounds Bromine Form Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Natural salt deposits and brines are the. Compounds Bromine Form.
From dir.indiamart.com
Bromine Compound organobromides Latest Price, Manufacturers & Suppliers Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. This section lists some binary compounds with. Compounds Bromine Form.
From www.numerade.com
SOLVEDPhosphorus (P) and bromine (Br) form a compound. Predict the Compounds Bromine Form They form polar covalent bonds in. Bromine is too reactive to exist as a free element in nature. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. This section. Compounds Bromine Form.
From cednwvxm.blob.core.windows.net
Bromine Formula Of Compound at Anthony Ford blog Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. They form polar covalent bonds in. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a. Compounds Bromine Form.
From exotclnfm.blob.core.windows.net
Bromine Ethyne Equation at William Spence blog Compounds Bromine Form They form polar covalent bonds in. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the. Compounds Bromine Form.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Compounds Bromine Form Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine is too reactive to exist as a free element in nature. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Instead, it occurs in compounds, the most common of which are sodium. Compounds Bromine Form.
From www.livescience.com
Facts About Bromine Live Science Compounds Bromine Form Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine is too reactive to exist as a free element in nature. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known. Compounds Bromine Form.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Compounds Bromine Form Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. They form polar covalent bonds in. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Natural. Compounds Bromine Form.
From exotclnfm.blob.core.windows.net
Bromine Ethyne Equation at William Spence blog Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. They form polar covalent bonds in. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine is too reactive to exist as a free element in nature. Bromine atoms. Compounds Bromine Form.
From joixmbukt.blob.core.windows.net
Bromine Compound With Oxygen at Virginia Driscoll blog Compounds Bromine Form They form polar covalent bonds in. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature.. Compounds Bromine Form.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Compounds Bromine Form Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. They form polar covalent bonds in. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine is too reactive to exist as a free element in nature. Bromine, chemical element, a deep red. Compounds Bromine Form.
From www.numerade.com
Bromine can form compounds or ions with any number of fluorine atoms Compounds Bromine Form They form polar covalent bonds in. Bromine is too reactive to exist as a free element in nature. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Instead, it occurs in compounds,. Compounds Bromine Form.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Compounds Bromine Form Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Natural salt deposits and brines are the main sources of. Instead, it occurs in compounds, the most common of which are sodium bromide. Compounds Bromine Form.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 Compounds Bromine Form Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Natural salt deposits and brines are the main sources of. They form polar covalent bonds in. Bromine is too reactive to exist as a free element. Compounds Bromine Form.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Compounds Bromine Form Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine is too reactive to exist as a free element in nature. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known. Compounds Bromine Form.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. They form polar covalent bonds in. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide. Compounds Bromine Form.
From techiescientist.com
11 Uses of Bromine That You Must Know Techiescientist Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen. Compounds Bromine Form.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Compounds Bromine Form They form polar covalent bonds in. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Natural salt deposits and brines are the main sources of. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine is too reactive to exist as a free element in. Compounds Bromine Form.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Compounds Bromine Form Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Instead, it occurs. Compounds Bromine Form.
From dxofeotiz.blob.core.windows.net
Bromine A Compound at Dawn Bocanegra blog Compounds Bromine Form Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Natural salt deposits and brines are the main sources of. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. This section lists some binary compounds with halogens (known as. Compounds Bromine Form.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Compounds Bromine Form Natural salt deposits and brines are the main sources of. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of. Compounds Bromine Form.
From www.pinterest.co.uk
Bromination of alkenes with Br2. The upper picture is about the pure Compounds Bromine Form Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. They form polar covalent bonds in. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Natural salt deposits and brines are the main sources of. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known. Compounds Bromine Form.
From www.doubtnut.com
[Telugu] Explain why phenol with bromine water forms 2,4,6tribromophe Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. Bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Natural salt deposits and brines are the main sources of.. Compounds Bromine Form.
From www.dreamstime.com
Bromine Form Periodic Table of Elements Stock Illustration Compounds Bromine Form They form polar covalent bonds in. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Natural salt deposits and brines are the main sources of. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine atoms are highly electronegative, with an electronegativity of about. Compounds Bromine Form.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Natural salt deposits and brines are the main sources of. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table.. Compounds Bromine Form.
From images-of-elements.com
Chemical Elements Bromine Compounds Bromine Form Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine is too reactive to exist as a free element in nature. They form polar covalent bonds in. Bromine atoms are highly electronegative, with an electronegativity of about. Compounds Bromine Form.
From gradegorilla.com
Gradegorilla GCSE Chemistry Compounds Bromine Form Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature. Natural salt deposits and brines are the main sources of. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium. Compounds Bromine Form.
From www.numerade.com
SOLVED Nitrogen and bromine are both nonmetals and will combine to Compounds Bromine Form They form polar covalent bonds in. Bromine, chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 of the periodic table. Bromine is too reactive to exist as a free element in nature. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Instead, it occurs in compounds,. Compounds Bromine Form.
From www.coursehero.com
[Solved] Decide whether each pair of elements in the table below will Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. Natural salt deposits and brines are the main sources of. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. Instead, it occurs in compounds, the most common of which are sodium bromide (nabr) and potassium bromide (kbr). Bromine, a halogen element. Compounds Bromine Form.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Compounds Bromine Form Bromine is too reactive to exist as a free element in nature. They form polar covalent bonds in. Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and. This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen. Bromine, chemical element, a deep red noxious liquid, and a. Compounds Bromine Form.