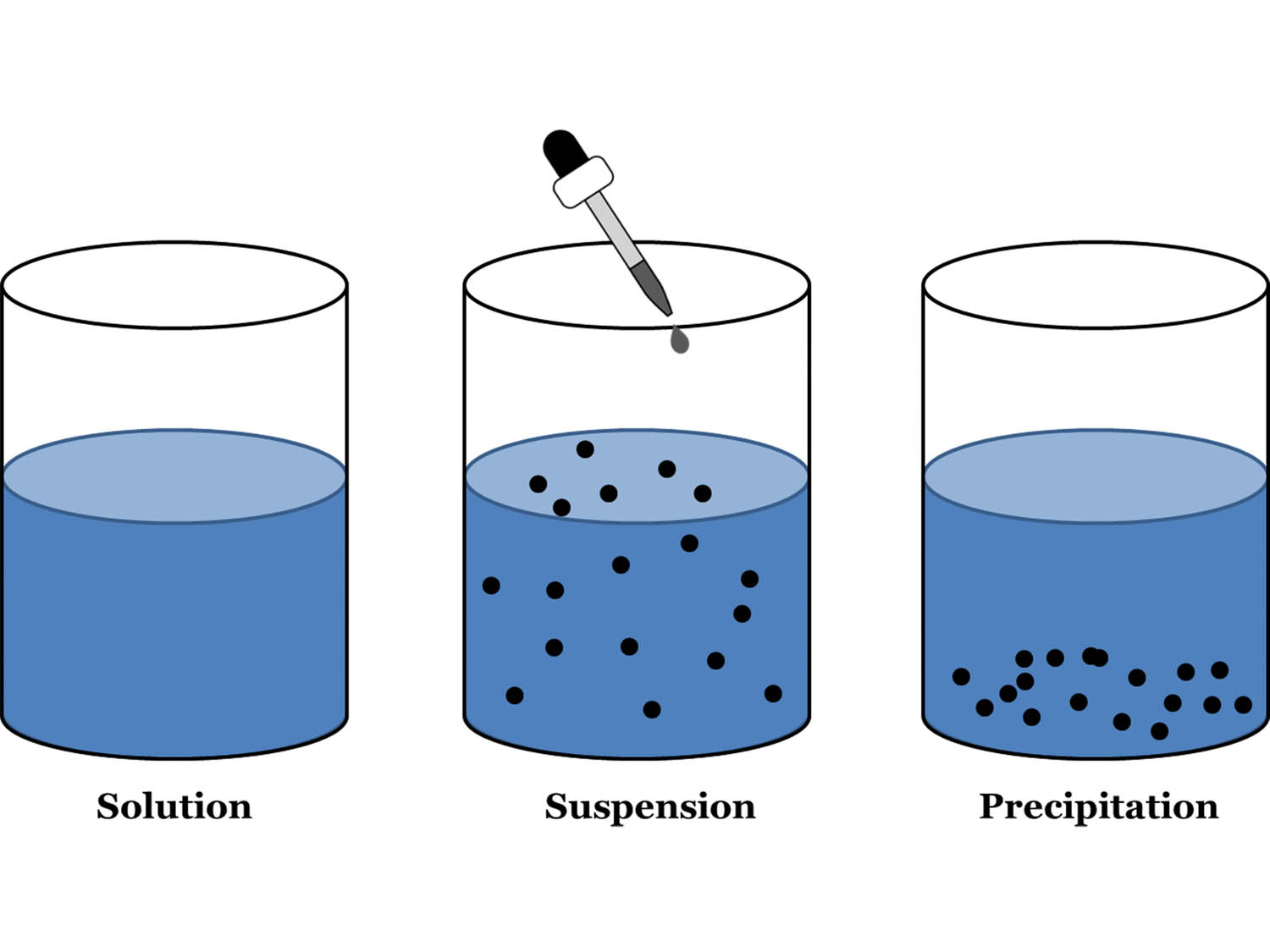
Suspension Definition Homogeneous . A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A solution is a homogeneous mixture where the solute particles are uniformly. A solution is a homogeneous mixture of two or more components. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. The substance that is dissolved is the solute. A homogeneous suspension means that if you. The dissolving agent is the solvent. In this type of mixture,. A suspension is when a solid component isn't dissolved in the solution. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger.
from byjus.com
Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A solution is a homogeneous mixture where the solute particles are uniformly. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. The substance that is dissolved is the solute. A homogeneous suspension means that if you.
Suspensions (Chemistry) Definition, Properties, Examples with Videos
Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. The substance that is dissolved is the solute. In this type of mixture,. A solution is a homogeneous mixture where the solute particles are uniformly. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A homogeneous suspension means that if you. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A solution is a homogeneous mixture of two or more components. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. The dissolving agent is the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is when a solid component isn't dissolved in the solution.
From www.slideshare.net
Properties of suspensions Suspension Definition Homogeneous Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. In this type of mixture,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000. Suspension Definition Homogeneous.
From www.pinterest.com
Suspension Easy Science Chemistry study guide, Physics concepts Suspension Definition Homogeneous In this type of mixture,. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. Both suspensions and colloids are heterogeneous mixtures, but the particle size in. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Suspension PowerPoint Presentation, free download ID4164419 Suspension Definition Homogeneous In this type of mixture,. The substance that is dissolved is the solute. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A solution is a homogeneous mixture of. Suspension Definition Homogeneous.
From slidetodoc.com
Solutions Homogeneous Mixtures M J Foster C W Suspension Definition Homogeneous A suspension is when a solid component isn't dissolved in the solution. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Suspensions and colloids are two common types of mixtures whose properties are in many ways. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Topics States of Matter Pure Substances Mixtures Physical and Suspension Definition Homogeneous A homogeneous suspension means that if you. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. In this type of mixture,. A solution is a homogeneous. Suspension Definition Homogeneous.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Suspension Definition Homogeneous Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture where the solute particles are uniformly. A solution is a homogeneous mixture of two or more components. The substance. Suspension Definition Homogeneous.
From www.youtube.com
Class 6 Science Chapter 7 Uses of solution, suspension uses properties Suspension Definition Homogeneous A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A homogeneous suspension means that if you. In this type of mixture,. A suspension is when a solid component isn't. Suspension Definition Homogeneous.
From slideplayer.com
Physical & Chemical Changes ppt download Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. The dissolving agent is the solvent. In this type of mixture,. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture of two or. Suspension Definition Homogeneous.
From mungfali.com
5 Examples Of Suspension Suspension Definition Homogeneous Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A homogeneous suspension means that if you. The dissolving agent is the solvent. The substance that is dissolved. Suspension Definition Homogeneous.
From www.expii.com
Suspensions — Definition & Examples Expii Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A homogeneous suspension means that if you. The substance that is dissolved is the solute. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A solution is. Suspension Definition Homogeneous.
From manualdatashockingly.z13.web.core.windows.net
Homogeneous Mixture With Dissolved Particles Suspension Definition Homogeneous Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A solution is a homogeneous mixture where the solute particles are uniformly. The substance that is dissolved is the solute. Solution and suspension are both types of mixtures, but they differ in their particle size and stability.. Suspension Definition Homogeneous.
From www.slideshare.net
Properties of suspensions Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Suspension Definition Homogeneous Solution and suspension are both types of mixtures, but they differ in their particle size and stability. The substance that is dissolved is the solute. The dissolving agent is the solvent. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture of two or more components. A suspension is defined as a. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Types of mixtures PowerPoint Presentation, free download ID4695980 Suspension Definition Homogeneous Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is defined. Suspension Definition Homogeneous.
From www.slideshare.net
Solutions, suspensions, and colloids Suspension Definition Homogeneous Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is when a solid component isn't dissolved in the solution. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A solution is a homogeneous mixture. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Composition of Matter PowerPoint Presentation, free download ID Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. The dissolving agent is the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is when a solid component isn't dissolved in the solution.. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Solution and Suspension PowerPoint Presentation, free download Suspension Definition Homogeneous Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A solution is a homogeneous mixture where the solute particles are uniformly. In this type of mixture,. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A suspension is defined as a homogenous mixture of particles. Suspension Definition Homogeneous.
From slideplayer.com
Learning Objectives Define the following terms mixture, solution Suspension Definition Homogeneous In this type of mixture,. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. The substance that is dissolved is the solute. A suspension is when a solid component isn't dissolved in the solution. A homogeneous suspension means that if you. A solution is a homogeneous. Suspension Definition Homogeneous.
From slideplayer.com
Learning Objectives Define the following terms mixture, solution Suspension Definition Homogeneous Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A solution is a homogeneous mixture where the solute particles are uniformly. The substance that is dissolved is the solute. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures.. Suspension Definition Homogeneous.
From stock.adobe.com
Solutions, suspension, precipitate. Solubility homogeneous mixture Suspension Definition Homogeneous In this type of mixture,. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture where the solute particles are uniformly. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension. Suspension Definition Homogeneous.
From www.slideserve.com
PPT 2.1 Classifying Matter PowerPoint Presentation, free download Suspension Definition Homogeneous A suspension is when a solid component isn't dissolved in the solution. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked. Suspension Definition Homogeneous.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. The dissolving agent is the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture of two or more components. A suspension is. Suspension Definition Homogeneous.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Definition Homogeneous A homogeneous suspension means that if you. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. In this type of mixture,. A solution is a homogeneous mixture where the solute particles are uniformly. A suspension is when a solid component isn't dissolved in the solution. The. Suspension Definition Homogeneous.
From pediaa.com
Difference Between Solution and Suspension Definition Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. The substance that is dissolved is the solute. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A solution is. Suspension Definition Homogeneous.
From slideplayer.com
Chemistry 11 Matter. ppt download Suspension Definition Homogeneous A suspension is when a solid component isn't dissolved in the solution. A solution is a homogeneous mixture where the solute particles are uniformly. A homogeneous suspension means that if you. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. Both suspensions and. Suspension Definition Homogeneous.
From kunduz.com
Homogeneous Mixtures Definition, Properties, Types, Examples Suspension Definition Homogeneous A homogeneous suspension means that if you. A suspension is when a solid component isn't dissolved in the solution. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A solution is a homogeneous mixture where the solute particles are uniformly. A suspension is defined as a homogenous mixture of particles with a. Suspension Definition Homogeneous.
From www.slideshare.net
Suspensions Formulation Overview Suspension Definition Homogeneous Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A solution is a homogeneous mixture where the solute particles are uniformly. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A solution is a homogeneous mixture of two or. Suspension Definition Homogeneous.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Definition Homogeneous A suspension is when a solid component isn't dissolved in the solution. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Both suspensions and colloids are heterogeneous mixtures, but. Suspension Definition Homogeneous.
From www.thoughtco.com
10 Heterogeneous and Homogeneous Mixtures Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. The dissolving agent is the solvent. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A solution. Suspension Definition Homogeneous.
From slideplayer.com
Learning Objectives Define the following terms mixture, solution Suspension Definition Homogeneous The dissolving agent is the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A homogeneous suspension means that if you. Suspensions and colloids are two common types of mixtures whose properties are in many. Suspension Definition Homogeneous.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Suspension Definition Homogeneous Solution and suspension are both types of mixtures, but they differ in their particle size and stability. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A solution is a homogeneous mixture where the solute particles are uniformly. In this type of mixture,.. Suspension Definition Homogeneous.
From collegedunia.com
Homogeneous Mixtures Solutions, Suspensions & Colloids Suspension Definition Homogeneous A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. The substance that is dissolved is the solute. A homogeneous suspension means that if you. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. In this. Suspension Definition Homogeneous.
From kunduz.com
Homogeneous Mixtures Definition, Properties, Types, Examples Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. A suspension is defined as a homogenous mixture of particles with a diameter greater than 1000 nm such that the particles are visible to naked eyes. A suspension is when a solid component isn't dissolved in the solution. Solution and suspension are both types of mixtures, but they. Suspension Definition Homogeneous.
From www.slideserve.com
PPT Compounds, Mixtures, & Solutions PowerPoint Presentation ID1842858 Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A solution is a homogeneous mixture of two or more components. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Suspensions and colloids are. Suspension Definition Homogeneous.
From ar.inspiredpencil.com
Example Of Suspension In Chemistry Suspension Definition Homogeneous A solution is a homogeneous mixture where the solute particles are uniformly. Solution and suspension are both types of mixtures, but they differ in their particle size and stability. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A homogeneous suspension means that if you. A. Suspension Definition Homogeneous.