Software Medical Device Labeling . Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. That fully integrate with existing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation.
from mavink.com
As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. That fully integrate with existing.
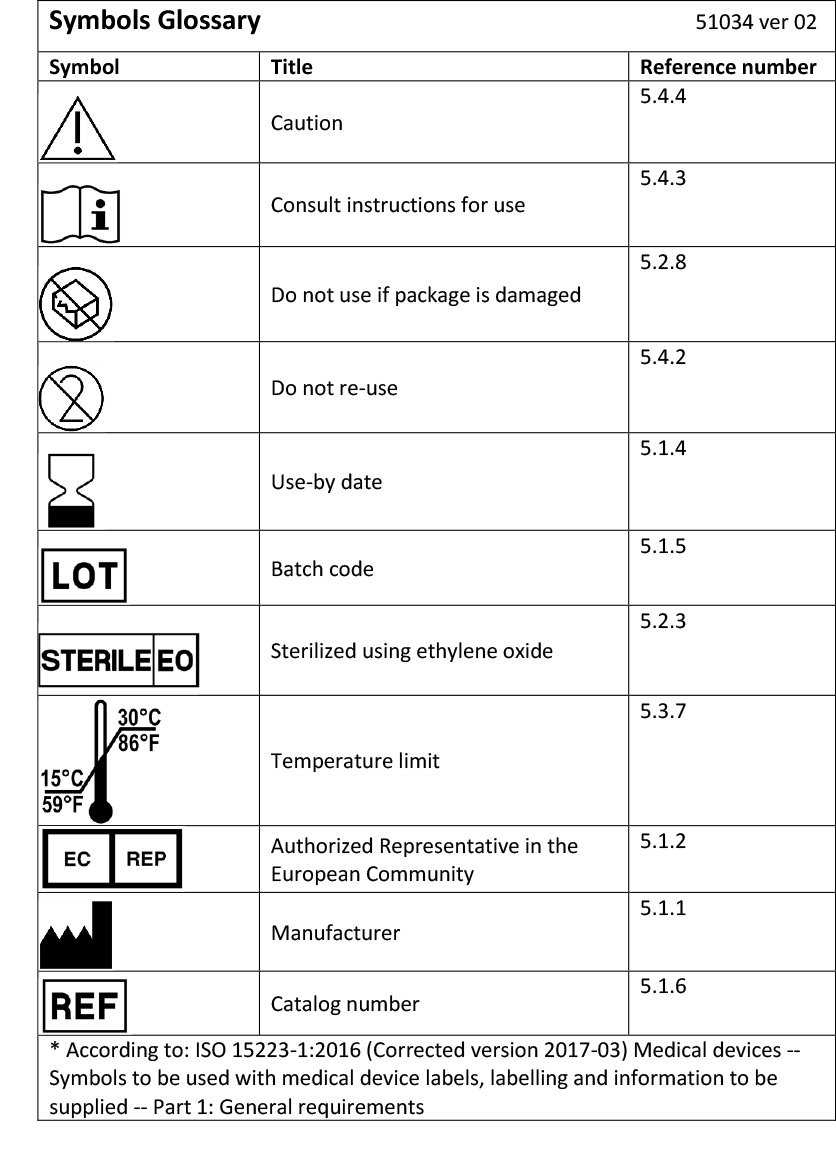
Medical Device Labeling Symbols
Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. That fully integrate with existing. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be.
From mavink.com
Medical Device Labeling Symbols Software Medical Device Labeling As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. That fully integrate with existing. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. This post will discuss what counts as a medical device label, where. Software Medical Device Labeling.
From www.neuralabel.com
Revolutionizing Medical Device Labeling in 5 Easy Areas with NeuraLabel Software Medical Device Labeling Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; That fully integrate with existing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The term software as a medical device is defined by the. Software Medical Device Labeling.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. That fully integrate with existing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. This guidance document describes the general. Software Medical Device Labeling.
From www.orielstat.com
Understanding FDA and EU Medical Device Labeling Requirements Oriel Software Medical Device Labeling This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. As a manufacturer of software as a medical device, you must ensure that you meet the relevant. Software Medical Device Labeling.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Software Medical Device Labeling Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. That fully integrate with. Software Medical Device Labeling.
From www.languageintelligence.com
5 Translating Tips for Medical Device Labeling Software Medical Device Labeling As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. That fully integrate with existing. In this blog, we summarize. Software Medical Device Labeling.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. That fully integrate with existing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. Then we will provide general guidelines. Software Medical Device Labeling.
From texaslabelprinters.com
Medical Device Label Printers UDI Label Printers Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; That. Software Medical Device Labeling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Software Medical Device Labeling As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. This post will discuss what counts as a medical device label, where they are required, and look at the. Software Medical Device Labeling.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The term software as a medical device is defined by the. Software Medical Device Labeling.
From mavink.com
Medical Device Labeling Symbols Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical. Software Medical Device Labeling.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. Then. Software Medical Device Labeling.
From datamyte.com
Medical Device Labeling A Comprehensive Guide DataMyte Software Medical Device Labeling The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. That fully integrate with existing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The term. Software Medical Device Labeling.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Software Medical Device Labeling That fully integrate with existing. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory. Software Medical Device Labeling.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. That fully integrate with existing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This guidance document describes the general. Software Medical Device Labeling.
From www.whaleteq.com
UDiBar_Medical Device Labeling Management Software_Products WhaleTeq Software Medical Device Labeling Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. As a manufacturer of software as. Software Medical Device Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; That fully integrate with existing. The term software as a medical device is defined by the. Software Medical Device Labeling.
From clin-r.com
Labels for Medical Devices Clin R Software Medical Device Labeling This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This post. Software Medical Device Labeling.
From www.teklynx.com
Why TEKLYNX is the Best Labeling Software for Medical Devices Software Medical Device Labeling That fully integrate with existing. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. This guidance document describes the general labelling principles. Software Medical Device Labeling.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. In this blog, we summarize what medical device software. Software Medical Device Labeling.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Software Medical Device Labeling The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. This guidance document describes the general labelling principles for medical devices and ivd medical devices and. Software Medical Device Labeling.
From medpak.com
Pharmaceutical & Medical Bar Code & Labeling Software Software Medical Device Labeling As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. Then we will provide general guidelines for producing labels that. Software Medical Device Labeling.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Software Medical Device Labeling The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This post. Software Medical Device Labeling.
From www.reedtech.com
UDI Labeling (Unique Device Identification) Best Practices Lexis Software Medical Device Labeling This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This post will discuss what counts as a medical device label, where they are required, and look at the key. Software Medical Device Labeling.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Software Medical Device Labeling Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. That fully integrate with existing. This post will. Software Medical Device Labeling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Software Medical Device Labeling The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. This guidance document. Software Medical Device Labeling.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Software Medical Device Labeling Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. In. Software Medical Device Labeling.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Software Medical Device Labeling That fully integrate with existing. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as. Software Medical Device Labeling.
From www.medtextpert.com
8 Facts Every Medical Software Developer Should Know About the MDR Software Medical Device Labeling That fully integrate with existing. This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. In this blog, we summarize what medical. Software Medical Device Labeling.
From software.boxuang.com
Medical Device Labeling Software Software Medical Device Labeling This guidance document describes the general labelling principles for medical devices and ivd medical devices and supersedes an earlier. Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. The term software as a medical device is defined by the international medical device regulators forum (imdrf) as software intended to be. Medical. Software Medical Device Labeling.
From www.teklynx.com
Why TEKLYNX is the Best Medical Device Labeling Software Software Medical Device Labeling In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. This post will discuss what counts as a medical device label, where they are required, and look at the. Software Medical Device Labeling.
From www.morningtrans.com
Best Practices for Medical Device Labeling Translations Morningside Software Medical Device Labeling Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms. Software Medical Device Labeling.
From blog.airlinehyd.com
Unlocking Expertise Free Online Training for Medical Device Labeling Software Medical Device Labeling This post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device labeling regulations. The eu mdr is designed to ensure public health and patient safety across europe and to increase quality and transparency of medical devices in. This guidance document describes the general labelling. Software Medical Device Labeling.
From satoasiapacific.com
SATO Medical Device Barcode Labelling Solution SATO AutoID Malaysia Software Medical Device Labeling That fully integrate with existing. As a manufacturer of software as a medical device, you must ensure that you meet the relevant regulatory requirements before placing. In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. The eu mdr is designed to ensure public health and patient safety across europe. Software Medical Device Labeling.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Software Medical Device Labeling Then we will provide general guidelines for producing labels that will benefit your customers and meet regulatory obligations. Medical device manufacturers require labeling and barcode software solutions that are secure, proven and reliable; In this blog, we summarize what medical device software (mdsw) manufacturers need to do in terms of udi under regulation. As a manufacturer of software as a. Software Medical Device Labeling.