Standard Heat Of Formation Pdf . Mallard, eds, nist chemistry webbook, nist. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. The heat of formation of any element in its standard state is defined as zero. Let's look at some common compounds and their standard enthalpies of formation: The standard enthalpy of reaction, ho, is the sum of the. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. ∗the standard entropy of the h+(aq) ion is defined to be 0. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their.
from www.doubtnut.com
All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of reaction, ho, is the sum of the. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Let's look at some common compounds and their standard enthalpies of formation: ∗the standard entropy of the h+(aq) ion is defined to be 0. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Mallard, eds, nist chemistry webbook, nist. The heat of formation of any element in its standard state is defined as zero. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a.
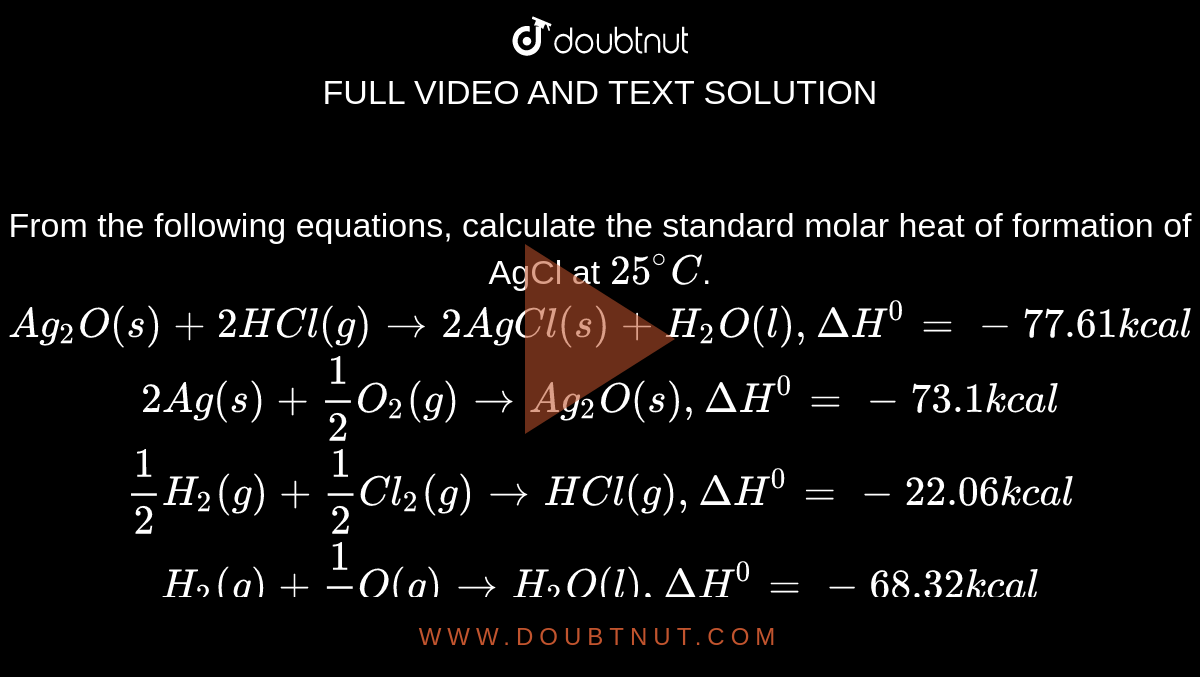
From the following equations, calculate the standard molar heat of
Standard Heat Of Formation Pdf Mallard, eds, nist chemistry webbook, nist. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The heat of formation of any element in its standard state is defined as zero. The standard enthalpy of reaction, ho, is the sum of the. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. All standard state, 25 °c and 1 bar (written to 1 decimal place). * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Mallard, eds, nist chemistry webbook, nist. Let's look at some common compounds and their standard enthalpies of formation: ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Pdf Mallard, eds, nist chemistry webbook, nist. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The standard enthalpy of reaction, ho, is the sum of the. ∗the standard entropy of the h+(aq) ion is defined to be 0. Let's look at some common compounds and their standard. Standard Heat Of Formation Pdf.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Heat Of Formation Pdf The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. ∗the standard entropy of the h+(aq) ion is defined to be 0. Let's look at some common compounds and their standard enthalpies. Standard Heat Of Formation Pdf.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. All standard state, 25 °c and 1 bar (written to 1. Standard Heat Of Formation Pdf.
From byjus.com
Standard heat of formation of ammonia is x kJ/mol.The heat of reaction Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. All standard state, 25 °c and 1 bar (written to 1. Standard Heat Of Formation Pdf.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Of Formation Pdf Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. ∗the standard entropy of the h+(aq) ion is defined to be 0. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Heat Of Formation Pdf Let's look at some common compounds and their standard enthalpies of formation: All standard state, 25 °c and 1 bar (written to 1 decimal place). The heat of formation of any element in its standard state is defined as zero. ∗the standard entropy of the h+(aq) ion is defined to be 0. Standard enthalpy of formation ( ∆η°f) definition the. Standard Heat Of Formation Pdf.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Pdf All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. Mallard, eds, nist chemistry webbook, nist. The standard enthalpy of reaction, ho, is the sum of the. Standard enthalpy of formation ( ∆η°f) definition the. Standard Heat Of Formation Pdf.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of reaction, ho, is the sum of the. Mallard, eds, nist chemistry webbook, nist. ∗the standard entropy of. Standard Heat Of Formation Pdf.
From mungfali.com
Standard Heat Formation Chart Standard Heat Of Formation Pdf ∗the standard entropy of the h+(aq) ion is defined to be 0. The heat of formation of any element in its standard state is defined as zero. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The standard enthalpy of reaction, ho, is the sum of the.. Standard Heat Of Formation Pdf.
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Heat Of Formation Pdf All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of reaction, ho, is the sum of the. ∗the standard entropy of the h+(aq) ion is defined to be 0. The heat of formation of any element in its standard state is defined as zero. The heat of formation or enthalpy of formation is. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Let's look at some common compounds and their standard enthalpies of formation: Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. ∗the standard entropy of the h+(aq) ion is defined. Standard Heat Of Formation Pdf.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Pdf The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The heat of formation of any element in its standard state is defined as zero. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure. Standard Heat Of Formation Pdf.
From www.doubtnut.com
From the following equations, calculate the standard molar heat of Standard Heat Of Formation Pdf The standard enthalpy of reaction, ho, is the sum of the. The heat of formation of any element in its standard state is defined as zero. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Mallard, eds, nist chemistry webbook, nist. Let's look. Standard Heat Of Formation Pdf.
From byjus.com
43. Calculate standard heat of formation of CS2. Given that standard Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. ∗the standard entropy of the h+(aq) ion is defined to be 0. Let's look at some common compounds. Standard Heat Of Formation Pdf.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Heat Of Formation Pdf The heat of formation of any element in its standard state is defined as zero. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The standard enthalpy of reaction, ho, is the sum of the. The heat of formation of any element in its standard state is defined as zero. ∗the standard entropy of the h+(aq) ion is defined to be 0. All standard state, 25 °c and. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. All standard state, 25 °c and 1 bar (written to 1 decimal place). The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The heat of formation of. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Mallard, eds, nist chemistry webbook, nist. All standard state, 25 °c and 1 bar (written to 1 decimal place). Let's look at some common compounds and their standard enthalpies of formation: Standard enthalpy of. Standard Heat Of Formation Pdf.
From oneclass.com
OneClass The standard heat of formation, Delta H_f degree, is defined Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with. Standard Heat Of Formation Pdf.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT CH 6 Thermochemistry PowerPoint Presentation, free download ID Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. * all standard enthalpy values are at 25°c, 1 molar concentration,. Standard Heat Of Formation Pdf.
From www.youtube.com
Standard Heat of Formation YouTube Standard Heat Of Formation Pdf The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The standard enthalpy of reaction, ho, is the sum of the. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. * all standard enthalpy. Standard Heat Of Formation Pdf.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. ∗the standard entropy of the h+(aq) ion is defined to be 0. The heat of formation of any element in its standard state is defined as zero. The standard enthalpy of reaction, ho, is the sum of the. All standard state, 25 °c and. Standard Heat Of Formation Pdf.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Mallard, eds, nist chemistry webbook, nist. Let's look at some common compounds and their standard enthalpies of formation: The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. Standard enthalpy of formation. Standard Heat Of Formation Pdf.
From quizzdbzdrelesccsj.z13.web.core.windows.net
Heat Of Formation Equations Standard Heat Of Formation Pdf The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. The heat of formation of any element in its standard state is defined as zero. The standard enthalpy of reaction, ho, is the sum of the. The heat of formation or enthalpy of formation is the standard heat. Standard Heat Of Formation Pdf.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Heat Of Formation Pdf The standard enthalpy of reaction, ho, is the sum of the. Let's look at some common compounds and their standard enthalpies of formation: The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of. Standard Heat Of Formation Pdf.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Heat Of Formation Pdf * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The standard enthalpy of reaction, ho, is the sum of the. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. Let's look at some common compounds and. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download Standard Heat Of Formation Pdf Mallard, eds, nist chemistry webbook, nist. The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. ∗the standard entropy of the h+(aq) ion is defined to be 0. All standard state, 25 °c and 1 bar (written to 1 decimal place). The heat of formation of any element. Standard Heat Of Formation Pdf.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Pdf The heat of formation of any element in its standard state is defined as zero. Let's look at some common compounds and their standard enthalpies of formation: * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Mallard, eds, nist chemistry webbook, nist. The standard enthalpy of reaction, ho, is the sum of the.. Standard Heat Of Formation Pdf.
From www.numerade.com
SOLVED a. Use standard heats of formation to determine the heat of Standard Heat Of Formation Pdf The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed. Standard Heat Of Formation Pdf.
From printablevascelomgm.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Pdf The heat of formation of any element in its standard state is defined as zero. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of reaction, ho, is the sum of the. The standard heat of formation. Standard Heat Of Formation Pdf.
From answerzonecarter.z13.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Pdf Mallard, eds, nist chemistry webbook, nist. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. The heat of formation or enthalpy of formation is the standard heat of reaction for forming one mole of product, starting from pure elements at their. The standard heat of formation. Standard Heat Of Formation Pdf.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Pdf Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of reaction, ho, is the sum of the. Let's look at some common compounds and their standard enthalpies of formation: ∗the. Standard Heat Of Formation Pdf.
From www.youtube.com
Std Heat of Formation versus Bond Energy YouTube Standard Heat Of Formation Pdf The standard heat of formation \(\left( \delta h^\text{o}_\text{f} \right)\) is the enthalpy change associated with the formation of one mole of a. * all standard enthalpy values are at 25°c, 1 molar concentration, and 1 atmosphere of pressure. The heat of formation of any element in its standard state is defined as zero. All standard state, 25 °c and 1. Standard Heat Of Formation Pdf.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Heat Of Formation Pdf Mallard, eds, nist chemistry webbook, nist. The heat of formation of any element in its standard state is defined as zero. The standard enthalpy of reaction, ho, is the sum of the. Standard enthalpy of formation ( ∆η°f) definition the enthalpy change when one mole of a compound is formed in its standard state from. * all standard enthalpy values. Standard Heat Of Formation Pdf.