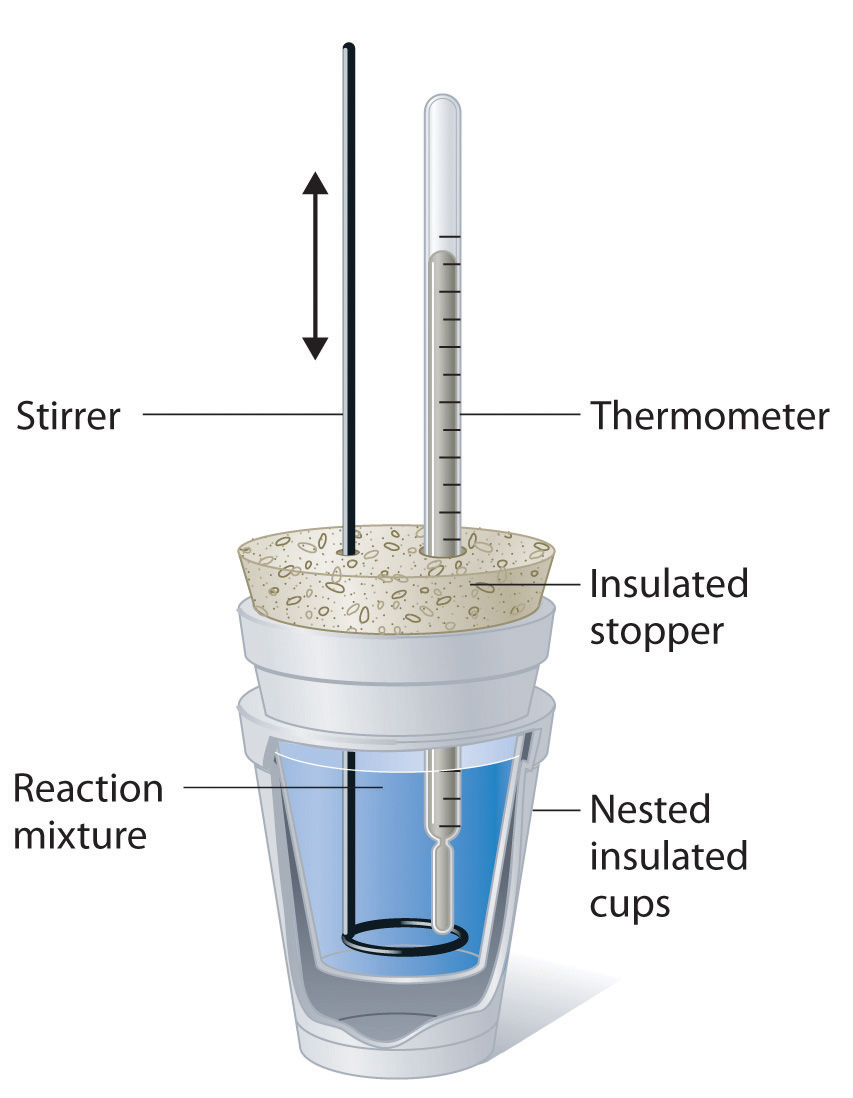
Calorimeter Unit Of Measurement . See how heat is calculated. It uses devices called calorimeters, which measure the change in. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. For example, when an exothermic. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. Calorimetry measures delta u & h. Calculate the molar heat of enthalpy for a reactions.
from chem.libretexts.org
Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Learn how heat is measured. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the change in. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry measures delta u & h. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. See how heat is calculated. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. For example, when an exothermic.
6.3 Calorimetry Chemistry LibreTexts
Calorimeter Unit Of Measurement Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry measures delta u & h. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate the molar heat of enthalpy for a reactions. See how heat is calculated. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. It uses devices called calorimeters, which measure the change in. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Learn how heat is measured.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. Calorimetry measures delta u & h. For example, when an exothermic. A calorimeter is a. Calorimeter Unit Of Measurement.
From schoolbag.info
Thermodynamics Review the Knowledge You Need to Score High 5 Steps Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat. Calorimeter Unit Of Measurement.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Unit Of Measurement Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Learn how heat is measured. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances. Calorimeter Unit Of Measurement.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Unit Of Measurement Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. See how heat is calculated. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is. Calorimeter Unit Of Measurement.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Unit Of Measurement Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. See how heat is calculated. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. A calorimeter. Calorimeter Unit Of Measurement.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Unit Of Measurement Calculate the molar heat of enthalpy for a reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. See how heat is calculated. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. Calorimetry is process of measuring amount of heat. Calorimeter Unit Of Measurement.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Unit Of Measurement Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Learn how heat is measured. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate the molar heat of enthalpy for a reactions. Calorimetry is the set of techniques used to measure enthalpy changes. Calorimeter Unit Of Measurement.
From study.com
Heat Measurement of Calorimeter Unit & Substances Lesson Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. For example, when an exothermic. Learn how heat is measured. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry is the set of. Calorimeter Unit Of Measurement.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter Unit Of Measurement Calculate the molar heat of enthalpy for a reactions. For example, when an exothermic. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is process of measuring amount of heat transfer in physical. Calorimeter Unit Of Measurement.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimeter Unit Of Measurement Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is process of measuring amount of heat transfer in physical. Calorimeter Unit Of Measurement.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Unit Of Measurement Learn how heat is measured. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. See how heat is calculated. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Unit Of Measurement.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Unit Of Measurement Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Learn how heat is measured. See how heat is calculated. For example, when an exothermic. A calorimeter is a device used to measure the amount of. Calorimeter Unit Of Measurement.
From www.youtube.com
How do you measure DeltaH? calorimetry YouTube Calorimeter Unit Of Measurement Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Learn how heat is measured. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. For example, when an exothermic. Calorimetry measures. Calorimeter Unit Of Measurement.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some. Calorimeter Unit Of Measurement.
From slideplayer.com
Calorimetry CP Unit 9 Chapter ppt download Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. See how heat is calculated. Learn how heat is measured. Calculate the molar heat of enthalpy for a reactions. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Unit Of Measurement.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Unit Of Measurement Calorimetry measures delta u & h. See how heat is calculated. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is process of. Calorimeter Unit Of Measurement.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Calorimeter Unit Of Measurement See how heat is calculated. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. For. Calorimeter Unit Of Measurement.
From shop.wf-education.com
Food Calorimeter Unit Calorimeter Unit Of Measurement Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. See how heat is calculated. For example, when an exothermic. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry measures delta u & h. It uses devices called calorimeters, which measure the change in.. Calorimeter Unit Of Measurement.
From www.philipharris.co.uk
Food Calorimeter B8H24816 Philip Harris Calorimeter Unit Of Measurement Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures delta u & h. It uses devices called calorimeters, which measure the change in. Calculate the molar heat of enthalpy for a reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Unit Of Measurement.
From gps-mech.blogspot.com
Mechanical Engineering Lab Manual for Steam and Power Generation Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Learn how heat is measured. Calorimetry measures delta u & h. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. See how heat is calculated. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the. Calorimeter Unit Of Measurement.
From www.studocu.com
Measurement of calorific value using bomb and Boys calorimeter Calorimeter Unit Of Measurement Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calculate the molar heat of enthalpy for a reactions. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. See how. Calorimeter Unit Of Measurement.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Unit Of Measurement Calculate the molar heat of enthalpy for a reactions. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry measures delta u & h. See how heat is calculated. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount. Calorimeter Unit Of Measurement.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Unit Of Measurement See how heat is calculated. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. For example, when an exothermic. It uses devices called calorimeters, which measure the change in. Calorimetry measures delta u & h. Learn how heat is measured. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Calorimeter Unit Of Measurement.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Unit Of Measurement Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. See how heat is calculated. For example, when an exothermic. Calculate the molar heat of enthalpy for a reactions. Understand. Calorimeter Unit Of Measurement.
From www.mdpi.com
Sensors Free FullText Indirect Calorimetry in Spontaneously Calorimeter Unit Of Measurement Learn how heat is measured. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calorimetry measures delta u & h. For example, when an exothermic. Calculate the molar heat of enthalpy for a reactions. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Understand the heat measurement unit,. Calorimeter Unit Of Measurement.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic Calorimeter Unit Of Measurement For example, when an exothermic. Calculate the molar heat of enthalpy for a reactions. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how heat is measured. Understand. Calorimeter Unit Of Measurement.
From www.researchgate.net
Diagram of a DØ calorimeter unit cell. Download Scientific Diagram Calorimeter Unit Of Measurement Learn how heat is measured. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry measures delta u & h. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using. Calorimeter Unit Of Measurement.
From saylordotorg.github.io
Calorimetry Calorimeter Unit Of Measurement Calorimetry measures delta u & h. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Learn how heat is measured. See how heat is calculated. Calorimetry is the set of techniques used to measure. Calorimeter Unit Of Measurement.
From www.pinterest.com
Class 10 ICSE Physics flash card for Calorimetry Units. . . physics Calorimeter Unit Of Measurement Learn how heat is measured. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. See how heat is calculated. It uses devices called calorimeters, which measure the change in. Calculate the molar heat of. Calorimeter Unit Of Measurement.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Unit Of Measurement Learn how heat is measured. Calculate the molar heat of enthalpy for a reactions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures delta u & h. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. See how heat is calculated. It. Calorimeter Unit Of Measurement.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimeter Unit Of Measurement Learn how heat is measured. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. See how heat is calculated. Calorimetry is process of measuring amount of heat transfer in physical changes, chemical reactions using calorimeter. Calculate heat, temperature change,. Calorimeter Unit Of Measurement.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Unit Of Measurement Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Learn how heat is measured. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. See how heat is calculated. Calculate the molar heat of enthalpy for a reactions. For example, when an exothermic. Calorimetry. Calorimeter Unit Of Measurement.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calculate the molar heat of enthalpy for a reactions. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calorimetry is process of measuring amount of heat transfer in physical. Calorimeter Unit Of Measurement.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Unit Of Measurement It uses devices called calorimeters, which measure the change in. Calorimetry measures delta u & h. For example, when an exothermic. Calculate the molar heat of enthalpy for a reactions. Understand the heat measurement unit, heat capacity of a calorimeter, and specific heat of some substances. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Calorimeter Unit Of Measurement.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Unit Of Measurement See how heat is calculated. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It uses devices called calorimeters, which measure the change in. For example, when an exothermic. Learn how heat is measured. Calculate the molar heat of enthalpy for a reactions. Understand the heat measurement unit, heat capacity of. Calorimeter Unit Of Measurement.