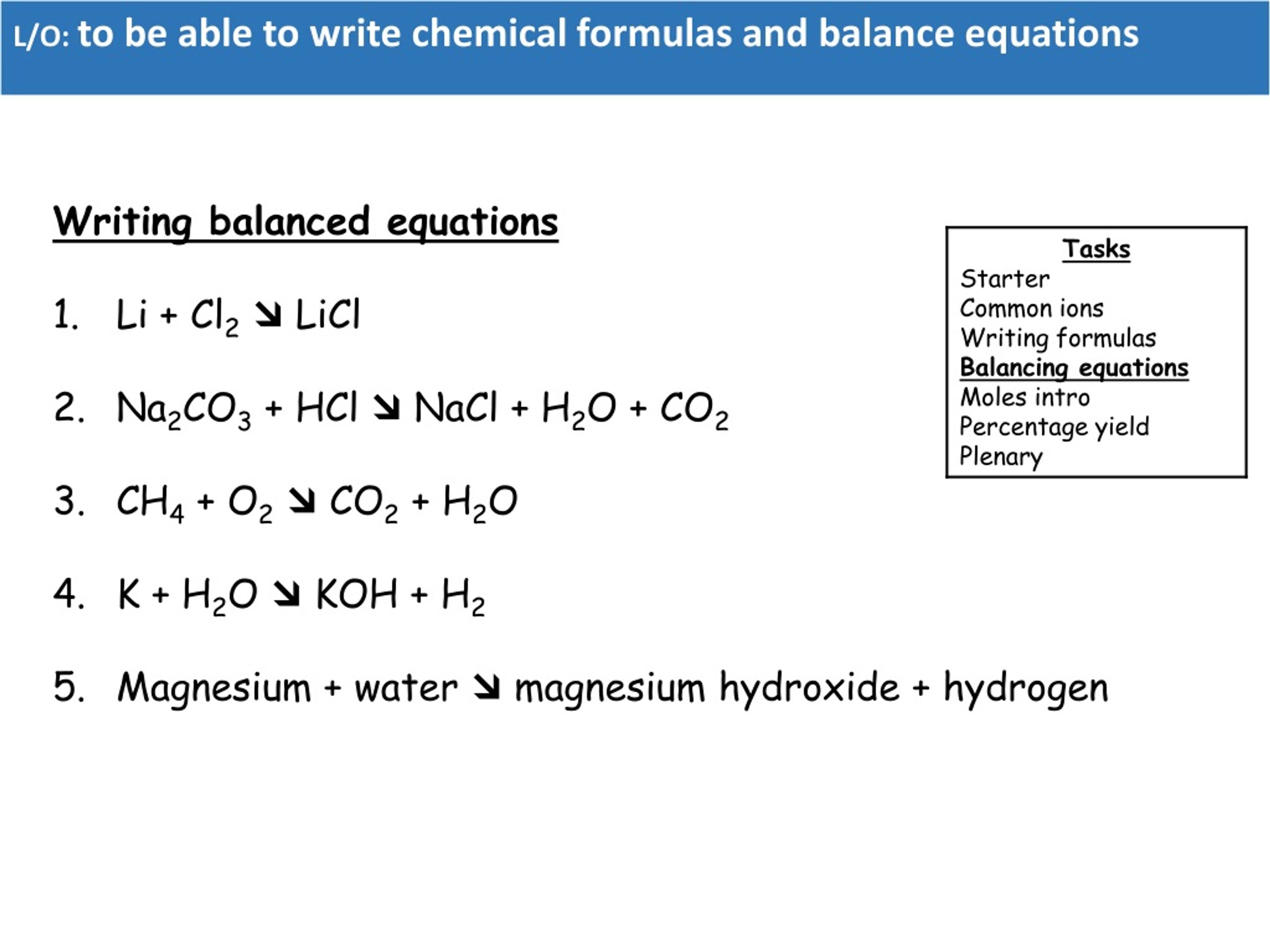
Chlorine Lithium Chloride Balanced Equation . this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium + chlorine = lithium chloride. Lithium chloride = chlorine + lithium. first, we set all coefficients to 1: Lithium + chlorine gas → lithium chloride. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. For each element, we check if the number of atoms is balanced on both. Licl = cl + li is a decomposition reaction where one mole of aqueous. This is a combination reaction. 1 licl = 1 cl 2 + 1 li. Li + cl 2 → licl. lithium chloride = lithium + dichlorine. — in this video we will balance the equation li + cl2 = licl and provide the.
from www.slideserve.com
This is a combination reaction. Lithium + chlorine gas → lithium chloride. first, we set all coefficients to 1: Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Licl = cl + li is a decomposition reaction where one mole of aqueous. 1 licl = 1 cl 2 + 1 li. For each element, we check if the number of atoms is balanced on both. lithium chloride = lithium + dichlorine. Lithium + chlorine = lithium chloride. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,.
PPT Formulas and equations PowerPoint Presentation, free download
Chlorine Lithium Chloride Balanced Equation lithium chloride = lithium + dichlorine. Lithium + chlorine = lithium chloride. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. — in this video we will balance the equation li + cl2 = licl and provide the. Licl = cl + li is a decomposition reaction where one mole of aqueous. This is a combination reaction. Lithium chloride = chlorine + lithium. first, we set all coefficients to 1: 1 licl = 1 cl 2 + 1 li. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Li + cl 2 → licl. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Lithium + chlorine gas → lithium chloride. For each element, we check if the number of atoms is balanced on both. lithium chloride = lithium + dichlorine.
From slideplayer.com
The Halogens F Cl Br I At. ppt download Chlorine Lithium Chloride Balanced Equation Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. This is a combination reaction. first, we set all coefficients to 1: — in this video we will balance the equation li + cl2 = licl and provide the. Licl = cl + li is a decomposition reaction where one mole. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Lewis Structure For LiCl How to Draw the Lewis Structure for LiCl Chlorine Lithium Chloride Balanced Equation Li + cl 2 → licl. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium chloride = chlorine + lithium. 1 licl = 1 cl 2 + 1 li. — in this video we will balance the equation li + cl2 = licl and provide the. For each element, we. Chlorine Lithium Chloride Balanced Equation.
From slideplayer.com
Chapter 8 Chemical Reactions ppt download Chlorine Lithium Chloride Balanced Equation Lithium + chlorine = lithium chloride. lithium chloride = lithium + dichlorine. 1 licl = 1 cl 2 + 1 li. first, we set all coefficients to 1: Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium chloride = chlorine + lithium. Licl = li + cl2 is a. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVEDWrite a skeleton equation for the reaction between lithium(s Chlorine Lithium Chloride Balanced Equation Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. — in this video we will balance the equation li + cl2 = licl and provide the. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. lithium chloride = lithium +. Chlorine Lithium Chloride Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Lithium Chloride Balanced Equation For each element, we check if the number of atoms is balanced on both. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Lithium + chlorine = lithium chloride. first, we set all coefficients to 1: Lithium chloride = chlorine + lithium. Licl = li + cl2 is a. Chlorine Lithium Chloride Balanced Equation.
From brainly.in
write down balanced equation for heated iron reacts with chlorine Chlorine Lithium Chloride Balanced Equation Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. 1 licl = 1 cl 2 + 1 li. For each element, we check if the number of atoms is balanced on both. This is a combination reaction. Lithium + chlorine = lithium chloride. Lithium chloride = chlorine + lithium. Li + cl =. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for the following chemical Chlorine Lithium Chloride Balanced Equation first, we set all coefficients to 1: lithium chloride = lithium + dichlorine. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. 1 licl = 1 cl 2 + 1 li. Lithium + chlorine = lithium chloride. Lithium chloride = chlorine + lithium. Lithium + chlorine gas →. Chlorine Lithium Chloride Balanced Equation.
From ar.inspiredpencil.com
Lithium Chloride Lewis Structure Chlorine Lithium Chloride Balanced Equation lithium chloride = lithium + dichlorine. This is a combination reaction. Lithium chloride = chlorine + lithium. — in this video we will balance the equation li + cl2 = licl and provide the. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Lithium + chlorine = lithium chloride. Licl =. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
How to Balance Li + Cl2 = LiCl (Lithium + Chlorine gas) YouTube Chlorine Lithium Chloride Balanced Equation Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. lithium chloride = lithium + dichlorine. first, we set all coefficients to 1: — in this video we will balance the equation li + cl2 = licl and provide the. Li + cl = licl is a synthesis reaction where one. Chlorine Lithium Chloride Balanced Equation.
From www.slideshare.net
Chemical Reactions Chlorine Lithium Chloride Balanced Equation Licl = cl + li is a decomposition reaction where one mole of aqueous. Lithium + chlorine = lithium chloride. Li + cl 2 → licl. This is a combination reaction. first, we set all coefficients to 1: Lithium + chlorine gas → lithium chloride. lithium chloride = lithium + dichlorine. Lithium chloride = chlorine + lithium. . Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chlorine Lithium Chloride Balanced Equation first, we set all coefficients to 1: Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Li + cl 2 → licl. Lithium + chlorine gas → lithium chloride. — in this video we will balance the equation li + cl2 = licl and provide the. Licl = cl +. Chlorine Lithium Chloride Balanced Equation.
From www.toppr.com
In passing chlorine gas through a concentrated solution of alkali, we Chlorine Lithium Chloride Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. — in this video we will balance the equation li + cl2 = licl and provide the. Licl = cl + li is a decomposition reaction where one mole of aqueous. Licl = li + cl2 is a decomposition reaction. Chlorine Lithium Chloride Balanced Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Lithium Chloride Balanced Equation first, we set all coefficients to 1: Lithium + chlorine = lithium chloride. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Licl = cl + li is a decomposition reaction where one mole of aqueous. Li + cl 2 → licl. This is a combination reaction. 1 licl = 1 cl. Chlorine Lithium Chloride Balanced Equation.
From www.slideserve.com
PPT Chapter 6 Ionic and Molecular Compounds PowerPoint Presentation Chlorine Lithium Chloride Balanced Equation — in this video we will balance the equation li + cl2 = licl and provide the. lithium chloride = lithium + dichlorine. For each element, we check if the number of atoms is balanced on both. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium chloride = chlorine. Chlorine Lithium Chloride Balanced Equation.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Lithium Chloride Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. first, we set all coefficients to 1: This is a combination reaction. Li + cl 2 → licl. Licl = cl + li is a decomposition reaction where one mole of aqueous. — in this video we will balance. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Equation for LiCl + H2O (Lithium chloride + Water) YouTube Chlorine Lithium Chloride Balanced Equation lithium chloride = lithium + dichlorine. Lithium + chlorine = lithium chloride. first, we set all coefficients to 1: Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Licl = cl +. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
Solid sodium chloride is obtained by the reaction of chlorine gas and Chlorine Lithium Chloride Balanced Equation This is a combination reaction. — in this video we will balance the equation li + cl2 = licl and provide the. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. first, we set all coefficients to 1: this net ionic equation indicates that solid silver chloride may be produced. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced equation for each of the following reactions Chlorine Lithium Chloride Balanced Equation Li + cl 2 → licl. This is a combination reaction. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Lithium chloride = chlorine + lithium. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. first, we set all coefficients to 1:. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Write the balanced equation for the. (i) Hydrogen + Chlorine → Hydrogen Chlorine Lithium Chloride Balanced Equation Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium + chlorine gas → lithium chloride. For each element, we check if the number of atoms is balanced on both. — in this video we will balance the equation li + cl2 = licl and provide the. 1 licl = 1. Chlorine Lithium Chloride Balanced Equation.
From dxookesmq.blob.core.windows.net
Chlorine Lithium Atom at Charles Camp blog Chlorine Lithium Chloride Balanced Equation Licl = cl + li is a decomposition reaction where one mole of aqueous. For each element, we check if the number of atoms is balanced on both. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Li + cl = licl is a synthesis reaction where one mole of. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for KI + Cl2 = KCl + I2 YouTube Chlorine Lithium Chloride Balanced Equation Lithium chloride = chlorine + lithium. 1 licl = 1 cl 2 + 1 li. For each element, we check if the number of atoms is balanced on both. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. this net ionic equation indicates that solid silver chloride may be produced from dissolved. Chlorine Lithium Chloride Balanced Equation.
From www.slideserve.com
PPT Unit 1C Chemical Equations & Reactions PowerPoint Presentation Chlorine Lithium Chloride Balanced Equation For each element, we check if the number of atoms is balanced on both. Lithium chloride = chlorine + lithium. Li + cl 2 → licl. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Licl = cl + li is a decomposition reaction where one mole of aqueous. . Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVED Enter a balanced chemical equation for the reaction between Chlorine Lithium Chloride Balanced Equation — in this video we will balance the equation li + cl2 = licl and provide the. Li + cl 2 → licl. first, we set all coefficients to 1: Lithium chloride = chlorine + lithium. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Licl = cl. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Lithium Chloride Balanced Equation Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. This is a combination reaction. lithium chloride = lithium + dichlorine. Li + cl 2 → licl. Lithium chloride = chlorine + lithium. Lithium + chlorine. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVED Solid lithium and chlorine gas are formed by the Chlorine Lithium Chloride Balanced Equation 1 licl = 1 cl 2 + 1 li. Licl = cl + li is a decomposition reaction where one mole of aqueous. Lithium chloride = chlorine + lithium. lithium chloride = lithium + dichlorine. Li + cl 2 → licl. This is a combination reaction. For each element, we check if the number of atoms is balanced on. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVED(a) When the metallic element lithium combines with the Chlorine Lithium Chloride Balanced Equation 1 licl = 1 cl 2 + 1 li. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. This is a combination reaction. Lithium + chlorine = lithium chloride. — in this. Chlorine Lithium Chloride Balanced Equation.
From www.toppr.com
Lithium Chloride Formula Definition, Concepts and Examples Chlorine Lithium Chloride Balanced Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. 1 licl = 1 cl 2 + 1 li. Lithium + chlorine = lithium chloride. Lithium + chlorine gas → lithium chloride. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. For each element,. Chlorine Lithium Chloride Balanced Equation.
From www.slideserve.com
PPT Formulas and equations PowerPoint Presentation, free download Chlorine Lithium Chloride Balanced Equation 1 licl = 1 cl 2 + 1 li. Lithium chloride = chlorine + lithium. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Lithium + chlorine = lithium chloride. — in this video we will balance the equation li + cl2 = licl and provide the. This is. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Li+Cl2=LiCl Balanced EquationLithium+Chlorine=Lithium chloride Chlorine Lithium Chloride Balanced Equation lithium chloride = lithium + dichlorine. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium chloride = chlorine + lithium. Licl = cl + li is a decomposition reaction where one. Chlorine Lithium Chloride Balanced Equation.
From slideplayer.com
Learning Objective Describe the properties of group one elements ppt Chlorine Lithium Chloride Balanced Equation Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Licl = cl + li is a decomposition reaction where one mole of aqueous. 1 licl = 1 cl 2 + 1 li. lithium chloride = lithium + dichlorine. For each element, we check if the number of atoms is balanced on. Chlorine Lithium Chloride Balanced Equation.
From slideplayer.com
Alkali Metals Electrostructure and reactivity Physical properties ppt Chlorine Lithium Chloride Balanced Equation For each element, we check if the number of atoms is balanced on both. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Li + cl 2 → licl. first, we set all coefficients to 1: this net ionic equation indicates that solid silver chloride may be produced from dissolved. Chlorine Lithium Chloride Balanced Equation.
From www.youtube.com
Cl2=ClO3^+Cl^ Balance the chemical equation by ion electron method or Chlorine Lithium Chloride Balanced Equation Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. lithium chloride = lithium + dichlorine. Lithium + chlorine gas → lithium chloride. — in this video we will balance the equation. Chlorine Lithium Chloride Balanced Equation.
From www.chegg.com
Solved 4. Write a balanced chemical equation for each of the Chlorine Lithium Chloride Balanced Equation Lithium + chlorine = lithium chloride. Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. For each element, we check if the number of atoms is balanced on both. Licl = cl + li is a decomposition reaction where one mole of aqueous. This is a combination reaction. Lithium chloride = chlorine +. Chlorine Lithium Chloride Balanced Equation.
From slideplayer.com
3/2/16 Today I will define chemical reactions and review formula Chlorine Lithium Chloride Balanced Equation Licl = li + cl2 is a decomposition reaction where two moles of aqueous lithium chloride. first, we set all coefficients to 1: Licl = cl + li is a decomposition reaction where one mole of aqueous. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium + chlorine = lithium. Chlorine Lithium Chloride Balanced Equation.
From www.numerade.com
SOLVEDQ When lithiur reacts with chlorine the following reaction Chlorine Lithium Chloride Balanced Equation Lithium + chlorine = lithium chloride. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Li + cl = licl is a synthesis reaction where one mole of lithium [li] and one. Lithium + chlorine gas → lithium chloride. Licl = li + cl2 is a decomposition reaction where two. Chlorine Lithium Chloride Balanced Equation.