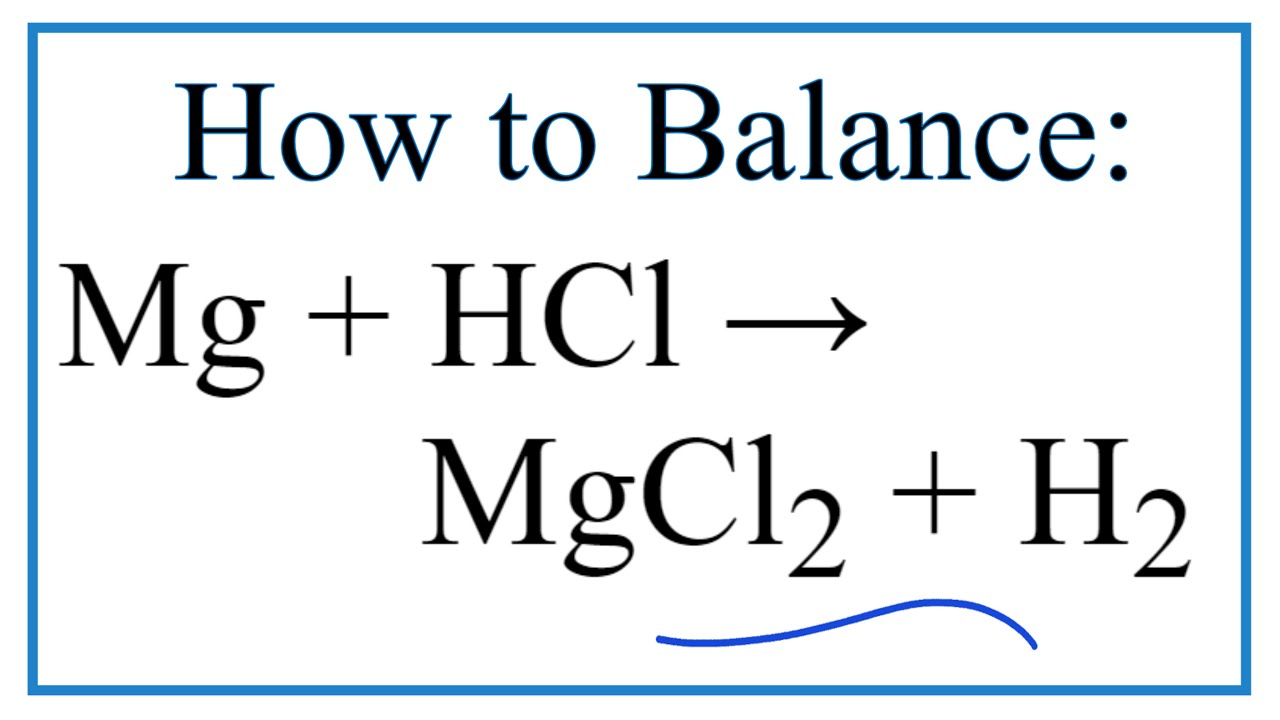
Limescale Hydrochloric Acid . Both samples are then subjected to ion exchange. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. It is classified as a class 8 dangerous good with highly corrosive properties. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. hydrochloric acid has traditionally been used to remove limescale. For toilets, stronger acids such as. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. Remember that calcium carbonate is used as an antacid, which means an. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. here are a few tips for limescale removal:
from mavink.com
For toilets, stronger acids such as. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. hydrochloric acid has traditionally been used to remove limescale. It is classified as a class 8 dangerous good with highly corrosive properties. Both samples are then subjected to ion exchange. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by.
Chemical Formula For Hydrochloric Acid
Limescale Hydrochloric Acid here are a few tips for limescale removal: Both samples are then subjected to ion exchange. here are a few tips for limescale removal: in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. Remember that calcium carbonate is used as an antacid, which means an. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. It is classified as a class 8 dangerous good with highly corrosive properties. hydrochloric acid has traditionally been used to remove limescale. For toilets, stronger acids such as.
From delphiseco.com
Delphis Eco Toilet & Limescale Cleaner PlantBased Free From Limescale Hydrochloric Acid — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Remember that calcium carbonate is used as an antacid, which means an. For toilets, stronger acids such as. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample. Limescale Hydrochloric Acid.
From www.researchgate.net
Limestone and dilute hydrochloric acid experiment scene 3. (a). Score Limescale Hydrochloric Acid For toilets, stronger acids such as. Remember that calcium carbonate is used as an antacid, which means an. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample. Limescale Hydrochloric Acid.
From delphiseco.com
Delphis Eco Toilet & Limescale Cleaner PlantBased Free From Limescale Hydrochloric Acid This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. hydrochloric acid has traditionally been used to remove limescale. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in. Limescale Hydrochloric Acid.
From www.alamy.com
Label on back of Tesco 100 limescale remover toilet gel danger Limescale Hydrochloric Acid here are a few tips for limescale removal: For toilets, stronger acids such as. It is classified as a class 8 dangerous good with highly corrosive properties. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. hydrochloric acid has. Limescale Hydrochloric Acid.
From byjus.com
Is hydrochloric acid strong or weak? Limescale Hydrochloric Acid For toilets, stronger acids such as. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: It is classified as a class 8 dangerous good with highly corrosive properties. Both samples are then subjected to ion exchange. — acids will react with the limescale to produce soluble metal. Limescale Hydrochloric Acid.
From www.anyrgb.com
Sodium Salts, acidity Function, efflorescence, acid Salt, Nitric acid Limescale Hydrochloric Acid in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. It is classified as a class 8 dangerous good with highly corrosive properties.. Limescale Hydrochloric Acid.
From delphiseco.com
Delphis Eco Toilet & Limescale Cleaner PlantBased Free From Limescale Hydrochloric Acid Both samples are then subjected to ion exchange. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. here are a few tips for limescale removal: — the best way to use hydrochloric acid for removing lime scale, in particular. Limescale Hydrochloric Acid.
From www.nycoproducts.com
Lime Scale Remover for Rust, Lime, Calcium, Foodservice NL008 Nyco Limescale Hydrochloric Acid hydrochloric acid has traditionally been used to remove limescale. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Both samples are then subjected. Limescale Hydrochloric Acid.
From acidsandbasesrios.weebly.com
pH Acids and Bases Limescale Hydrochloric Acid — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. here are a few tips for limescale removal: in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. Both samples are then subjected to ion. Limescale Hydrochloric Acid.
From www.alamy.com
Label on back of Tesco 100 limescale remover toilet gel danger Limescale Hydrochloric Acid For toilets, stronger acids such as. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. Remember. Limescale Hydrochloric Acid.
From www.ttbsupplies.com
S7 Limescale Remover 750ml ORCA Orca Hygiene Limescale Hydrochloric Acid here are a few tips for limescale removal: It is classified as a class 8 dangerous good with highly corrosive properties. hydrochloric acid has traditionally been used to remove limescale. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison.. Limescale Hydrochloric Acid.
From collegedunia.com
Uses Of Hydrochloric Acid Definition, Physical & Chemical Properties Limescale Hydrochloric Acid This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. hydrochloric acid has traditionally been used to remove limescale. For toilets, stronger acids such as. Both samples are then subjected to ion exchange. — acids will react with the limescale. Limescale Hydrochloric Acid.
From www.reddit.com
how to get these outdoor tiles looking new again? looks like limescale Limescale Hydrochloric Acid Remember that calcium carbonate is used as an antacid, which means an. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. It is classified as a class 8 dangerous good. Limescale Hydrochloric Acid.
From hg.eu
HG limescale remover concentrate Thé strong limescale cleaner Limescale Hydrochloric Acid here are a few tips for limescale removal: hydrochloric acid has traditionally been used to remove limescale. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. For toilets, stronger acids such as. It is classified as a class 8. Limescale Hydrochloric Acid.
From delphiseco.com
Delphis Eco Toilet & Limescale Cleaner PlantBased Free From Limescale Hydrochloric Acid — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. Remember that calcium carbonate is used as an antacid,. Limescale Hydrochloric Acid.
From dxoglbyfw.blob.core.windows.net
Hydrochloric Acid For Water Treatment at Sharon Clark blog Limescale Hydrochloric Acid Remember that calcium carbonate is used as an antacid, which means an. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of. Limescale Hydrochloric Acid.
From www.youtube.com
How to write the formula for Hydrochloric acid (HCl) YouTube Limescale Hydrochloric Acid Remember that calcium carbonate is used as an antacid, which means an. It is classified as a class 8 dangerous good with highly corrosive properties. here are a few tips for limescale removal: This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water. Limescale Hydrochloric Acid.
From wynnfraser.co.nz
Hydrochloric Acid Etch 33 4Ltr Wynn Fraser Limescale Hydrochloric Acid This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. hydrochloric acid has traditionally been used to remove limescale. Both samples. Limescale Hydrochloric Acid.
From www.chemicals.co.uk
The Science Behind Hydrochloric Acid The Chemistry Blog Limescale Hydrochloric Acid It is classified as a class 8 dangerous good with highly corrosive properties. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water. Limescale Hydrochloric Acid.
From www.dreamstime.com
Drawn Molecule and Formula of Hydrochloric Acid Stock Vector Limescale Hydrochloric Acid — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: — acids will react with the limescale to produce soluble metal salts which. Limescale Hydrochloric Acid.
From dengarden.com
How to Remove Limescale With Vinegar or Lemon Juice Dengarden Limescale Hydrochloric Acid It is classified as a class 8 dangerous good with highly corrosive properties. Remember that calcium carbonate is used as an antacid, which means an. hydrochloric acid has traditionally been used to remove limescale. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by.. Limescale Hydrochloric Acid.
From www.alamy.com
Hydrochloric Acid High Resolution Stock Photography and Images Alamy Limescale Hydrochloric Acid It is classified as a class 8 dangerous good with highly corrosive properties. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. This water. Limescale Hydrochloric Acid.
From www.domestos.com
Domestos Zero Limescale Toilet Gel Domestos Limescale Hydrochloric Acid — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. Remember that calcium carbonate is used as an antacid, which means an. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of. Limescale Hydrochloric Acid.
From www.compoundchem.com
Compound Interest The Chemistry of Limescale Limescale Hydrochloric Acid Remember that calcium carbonate is used as an antacid, which means an. It is classified as a class 8 dangerous good with highly corrosive properties. hydrochloric acid has traditionally been used to remove limescale. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. This water is then boiled to. Limescale Hydrochloric Acid.
From mstimms-gcse.blogspot.com
Ms Timms GCSE Limestone reaction cycle chemistry Limescale Hydrochloric Acid For toilets, stronger acids such as. Remember that calcium carbonate is used as an antacid, which means an. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. Both samples are then subjected to ion exchange. here are a few tips for limescale removal:. Limescale Hydrochloric Acid.
From delphiseco.com
Delphis Eco Heavy Duty Toilet Cleaner & Limescale Remover PlantBased Limescale Hydrochloric Acid — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. Remember that calcium carbonate is used as an antacid, which means an.. Limescale Hydrochloric Acid.
From dokumen.tips
(PDF) PRODUCT SAFETY DATA SHEET Viking Direct · HARPIC 100 Limescale Limescale Hydrochloric Acid in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. hydrochloric acid has traditionally been used to remove limescale. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. — the best way to use hydrochloric acid for removing. Limescale Hydrochloric Acid.
From delphiseco.com
Delphis Eco Toilet & Limescale Cleaner PlantBased Free From Limescale Hydrochloric Acid This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. For toilets, stronger acids such as. Both samples are then subjected to ion. Limescale Hydrochloric Acid.
From kimmachem.com
Hydrochloric Acid HCL Xilong Trung Quốc Limescale Hydrochloric Acid This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. — acids will react with the limescale to produce soluble metal salts which can then simply be washed away. It is classified as a class 8 dangerous good with highly corrosive. Limescale Hydrochloric Acid.
From alliancechemical.com
Hydrochloric Acid 37 (HCL 37) Technical Grade Alliance Chemical Limescale Hydrochloric Acid It is classified as a class 8 dangerous good with highly corrosive properties. Remember that calcium carbonate is used as an antacid, which means an. For toilets, stronger acids such as. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. — acids will react with the limescale to produce. Limescale Hydrochloric Acid.
From www.hunker.com
How to Remove Lime Scale With Hydrochloric Acid Hunker Limescale Hydrochloric Acid Remember that calcium carbonate is used as an antacid, which means an. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. in this experiment, students observe as the teacher prepares temporarily hard water by bubbling carbon dioxide through limewater. It is classified as. Limescale Hydrochloric Acid.
From sciencenotes.org
Hydrochloric Acid Science Notes and Projects Limescale Hydrochloric Acid here are a few tips for limescale removal: This water is then boiled to leave a solid residue, which can then be tested using hydrochloric acid, before boiling a sample of permanently hard water for comparison. — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an. Limescale Hydrochloric Acid.
From mavink.com
Chemical Formula For Hydrochloric Acid Limescale Hydrochloric Acid — the best way to use hydrochloric acid for removing lime scale, in particular the heavier deposits, has been explained in an article by. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: — acids will react with the limescale to produce soluble metal salts which. Limescale Hydrochloric Acid.
From edu.rsc.org
Hydrochloric acid Magnificent molecules RSC Education Limescale Hydrochloric Acid hydrochloric acid has traditionally been used to remove limescale. Remember that calcium carbonate is used as an antacid, which means an. here are a few tips for limescale removal: It is classified as a class 8 dangerous good with highly corrosive properties. For toilets, stronger acids such as. — the best way to use hydrochloric acid for. Limescale Hydrochloric Acid.