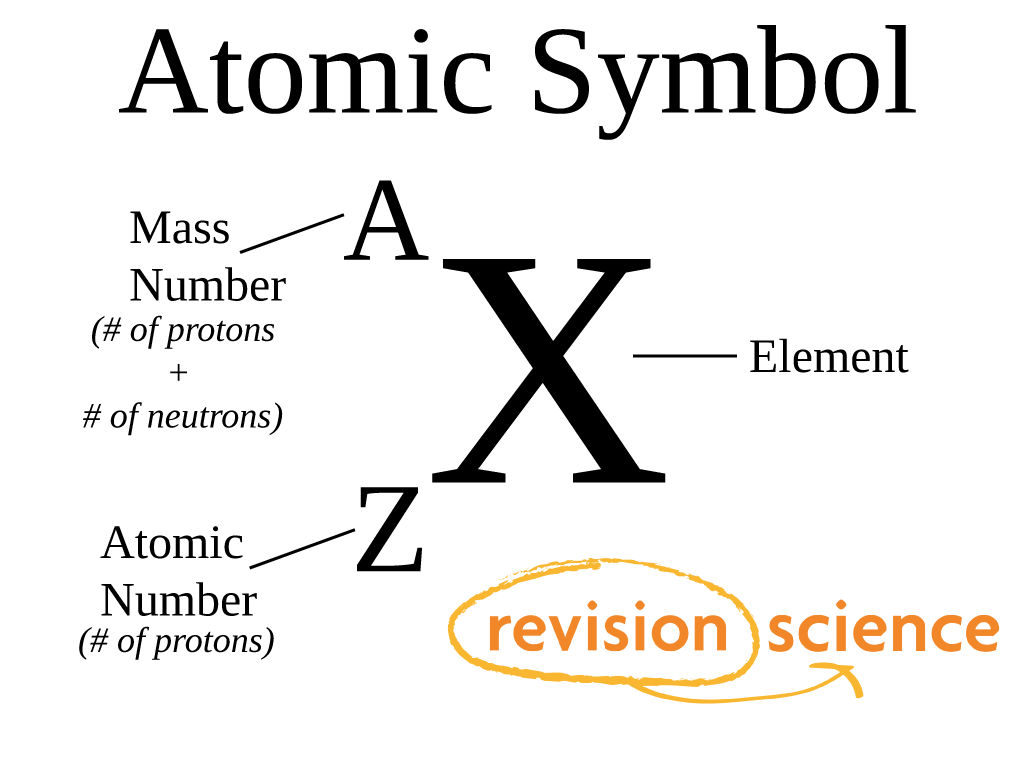
Atomic Number Of An Element X Is 19 . The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. Let's solve the question step by step: A) configuration of x (19)=2,8,8,1. The list is ordered by increasing atomic number, which is the number of. The number of electrons in its ion x + will be: The atomic number of an element x is 19. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: (a) 18 (b) 19 (c) 20 (d) 21 B) it belongs to fourth period and its valency is 1. Determine the electronic configuration of element 'x' with atomic number 19. The atomic number of an element 'x' is 19. The atomic number of an element x is 19.
from nabighreswara.blogspot.com
The atomic number of an element 'x' is 19. B) it belongs to fourth period and its valency is 1. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 The atomic number of an element x is 19. (ii) to which period of the modern periodic table does. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. A) configuration of x (19)=2,8,8,1. (i) write its electronic configuration. Let's solve the question step by step:
Atomic Number For Gold Nabigh Reswara
Atomic Number Of An Element X Is 19 (ii) to which period of the modern periodic table does. (a) 18 (b) 19 (c) 20 (d) 21 Let's solve the question step by step: A) configuration of x (19)=2,8,8,1. B) it belongs to fourth period and its valency is 1. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. Determine the electronic configuration of element 'x' with atomic number 19. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 The atomic number of an element x is 19. (ii) to which period of the modern periodic table does. The atomic number of an element x is 19. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: (i) write its electronic configuration. The list is ordered by increasing atomic number, which is the number of. The atomic number of an element x is 19.
From utedzz.blogspot.com
Periodic Table With Element Names Hd Periodic Table Timeline Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. B) it belongs to fourth period and its valency is 1. (ii) to which period of the modern periodic table does. A) configuration of x (19)=2,8,8,1. The atomic number of an element x is 19. The atomic number of an element 'x' is 19. Let's solve the question step by step:. Atomic Number Of An Element X Is 19.
From www.teachmint.com
Name Of Element Physics Notes Teachmint Atomic Number Of An Element X Is 19 B) it belongs to fourth period and its valency is 1. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 (ii) to which period of the modern periodic table does. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. (a) 18 (b) 19 (c) 20 (d) 21 The. Atomic Number Of An Element X Is 19.
From www.worksheetsplanet.com
What is the Atomic Number? Worksheets Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. (a) 20 (b) 19 (c) 18 (d) 16 the element. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: B) it belongs to fourth period and its valency is 1. The number of. Atomic Number Of An Element X Is 19.
From chemassist.blogspot.com
ChemAssist Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. B) it belongs to fourth period and its valency is 1. Determine the electronic configuration of element 'x' with atomic number 19. A) configuration of x (19)=2,8,8,1. The atomic number of an element x is 19. The number of electrons in its ion x + will be: The. Atomic Number Of An Element X Is 19.
From www.toppr.com
46. The atomic number of an element X is 19. The number of electrons in Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. B) it belongs to fourth period and its valency is 1. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: The list is ordered by increasing atomic number, which is. Atomic Number Of An Element X Is 19.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Atomic Number Of An Element X Is 19 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. (a) 18 (b) 19 (c) 20 (d) 21 Let's solve the question step by step: The atomic number of an element x is 19. The atomic number of an element 'x' is 19. B) it belongs to fourth period and its valency. Atomic Number Of An Element X Is 19.
From www.doubtnut.com
Three elements 'X', 'Y' and 'Z' have atomic numbers 18, 19 and 20 resp Atomic Number Of An Element X Is 19 The list is ordered by increasing atomic number, which is the number of. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 The number of electrons in its ion x + will be: (i) write its electronic configuration. The atomic number of an element x is 19. (a) 18 (b) 19. Atomic Number Of An Element X Is 19.
From sustainablegros.weebly.com
Periodic table atomic number superscript sustainableGros Atomic Number Of An Element X Is 19 (i) write its electronic configuration. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number of an element x is 19. Determine the electronic configuration of element 'x' with atomic number 19. If the number of protons in one atom of an element y is 20, then the number. Atomic Number Of An Element X Is 19.
From byjus.com
The minimum atomic no. of an element in which number of s elecron Atomic Number Of An Element X Is 19 (ii) to which period of the modern periodic table does. The number of electrons in its ion x + will be: Determine the electronic configuration of element 'x' with atomic number 19. The number of electrons in its ion x + will be: The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21. Atomic Number Of An Element X Is 19.
From utedzz.blogspot.com
Periodic Table Chemistry Atomic Number And Mass Number Periodic Table Atomic Number Of An Element X Is 19 (a) 20 (b) 19 (c) 18 (d) 16 the element. Determine the electronic configuration of element 'x' with atomic number 19. The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. (i) write its electronic configuration. If the number of protons. Atomic Number Of An Element X Is 19.
From www.youtube.com
How to Find the Atomic Number on the Periodic Table YouTube Atomic Number Of An Element X Is 19 A) configuration of x (19)=2,8,8,1. The atomic number of an element x is 19. B) it belongs to fourth period and its valency is 1. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. (a) 20 (b) 19 (c) 18 (d) 16 the element. (a) 18 (b) 19 (c) 20 (d) 21 Let's solve the question step. Atomic Number Of An Element X Is 19.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Atomic Number Of An Element X Is 19 B) it belongs to fourth period and its valency is 1. The atomic number of an element x is 19. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: A) configuration of x (19)=2,8,8,1. Determine the electronic configuration of element 'x' with. Atomic Number Of An Element X Is 19.
From byjus.com
the number of electron in a neutral atom of an element is equal to a Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. Let's solve the question step by step: (a) 20 (b) 19 (c) 18 (d) 16 the element. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: Determine the electronic configuration of element 'x'. Atomic Number Of An Element X Is 19.
From www.adda247.com
Atomic Mass of Elements 1 to 30 with Symbols PDF Download Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 B) it belongs to fourth period and its valency is 1. The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. The atomic number of an element x is 19. A) configuration of x (19)=2,8,8,1. The atomic number of an element. Atomic Number Of An Element X Is 19.
From courses.lumenlearning.com
The Periodic Table of Elements Biology for Majors I Atomic Number Of An Element X Is 19 The number of electrons in its ion x + will be: The atomic number of an element 'x' is 19. (a) 18 (b) 19 (c) 20 (d) 21 If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: (a) 18 (b) 19 (c). Atomic Number Of An Element X Is 19.
From www.sliderbase.com
The Atom Presentation Chemistry Atomic Number Of An Element X Is 19 (i) write its electronic configuration. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number of an element x is 19. The atomic number of an element x is 19. (ii) to which period of the modern periodic table does. B) it belongs to fourth period and its valency. Atomic Number Of An Element X Is 19.
From sciencenotes.org
List of Elements By Atomic Number Atomic Number Of An Element X Is 19 (i) write its electronic configuration. Let's solve the question step by step: Determine the electronic configuration of element 'x' with atomic number 19. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: The atomic number of an element x is 19. The. Atomic Number Of An Element X Is 19.
From chemistry.about.com
Element List Atomic Number, Element Name and Symbol Atomic Number Of An Element X Is 19 (ii) to which period of the modern periodic table does. (a) 18 (b) 19 (c) 20 (d) 21 The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. (i) write its electronic configuration. The. Atomic Number Of An Element X Is 19.
From periodictable.me
Printable Periodic Table With Atomic Number Atomic Number Of An Element X Is 19 The list is ordered by increasing atomic number, which is the number of. B) it belongs to fourth period and its valency is 1. The atomic number of an element 'x' is 19. (ii) to which period of the modern periodic table does. Determine the electronic configuration of element 'x' with atomic number 19. The number of electrons in its. Atomic Number Of An Element X Is 19.
From www.vectorstock.com
Periodic table of the elements with atomic number Vector Image Atomic Number Of An Element X Is 19 The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 A) configuration of x (19)=2,8,8,1. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: The number of electrons in its ion x + will. Atomic Number Of An Element X Is 19.
From brainly.in
an element X has atomic mass 27 and atomic number 13 first draw the Atomic Number Of An Element X Is 19 The number of electrons in its ion x + will be: (a) 18 (b) 19 (c) 20 (d) 21 If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: 119 rows here is a list of elements of the periodic table, their atomic. Atomic Number Of An Element X Is 19.
From brainly.in
The atomic number of an element x is 12 (1) identify element x and Atomic Number Of An Element X Is 19 If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be: The atomic number of an element x is 19. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. A) configuration of x (19)=2,8,8,1. (a). Atomic Number Of An Element X Is 19.
From ar.inspiredpencil.com
Atomic Number Of Elements Atomic Number Of An Element X Is 19 Determine the electronic configuration of element 'x' with atomic number 19. The number of electrons in its ion x + will be: (ii) to which period of the modern periodic table does. Let's solve the question step by step: (i) write its electronic configuration. The atomic number of an element x is 19. (a) 20 (b) 19 (c) 18 (d). Atomic Number Of An Element X Is 19.
From www.youtube.com
20153MarksThe atomic number of an element 'X' is 19. (a) Write its Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. B) it belongs to fourth period and its valency is 1. (ii) to which period of the modern periodic table does. (a) 18 (b) 19 (c) 20 (d) 21 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The number of electrons. Atomic Number Of An Element X Is 19.
From periodictable.me
Printable Periodic Table With Atomic Number Atomic Number Of An Element X Is 19 (a) 20 (b) 19 (c) 18 (d) 16 the element. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The number of electrons in its ion x + will be: The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this.. Atomic Number Of An Element X Is 19.
From www.youtube.com
The atomic number of an element X is 19. The number of electrons in its Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. The atomic number of an element x is 19. The atomic number of an element x is 19. Let's solve the question step by step: (a) 20 (b) 19 (c) 18 (d) 16 the element. If the number of protons in one atom of an element y is. Atomic Number Of An Element X Is 19.
From www.geeksforgeeks.org
Differences Between Atomic Mass and Atomic Number Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 Determine the electronic configuration of element 'x' with atomic number 19. (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. If the number of protons in one atom of an. Atomic Number Of An Element X Is 19.
From nabighreswara.blogspot.com
Atomic Number For Gold Nabigh Reswara Atomic Number Of An Element X Is 19 (i) write its electronic configuration. The number of electrons in its ion x + will be: 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will. Atomic Number Of An Element X Is 19.
From www.chegg.com
Solved The atomic number of an element is determined by the Atomic Number Of An Element X Is 19 Let's solve the question step by step: Determine the electronic configuration of element 'x' with atomic number 19. A) configuration of x (19)=2,8,8,1. (ii) to which period of the modern periodic table does. (a) 18 (b) 19 (c) 20 (d) 21 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. If the number of protons in one. Atomic Number Of An Element X Is 19.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Atomic Number Of An Element X Is 19 The atomic number of an element x is 19. The atomic number of an element 'x' is 19. The number of electrons in its ion x + will be: (i) write its electronic configuration. The atomic number of an element x is 19. If the number of protons in one atom of an element y is 20, then the number. Atomic Number Of An Element X Is 19.
From periodictable.me
How To Find Element Atomic Number, Element Name & Symbol Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 The number of electrons in its ion x + will be: The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. The atomic number of an element 'x' is 19. B) it. Atomic Number Of An Element X Is 19.
From www.researchgate.net
As the figure shows, the atomic number of an element is determined Atomic Number Of An Element X Is 19 (a) 18 (b) 19 (c) 20 (d) 21 50% students answered this. (ii) to which period of the modern periodic table does. The number of electrons in its ion x+ will be (a) 18 (b) 19 (c) 20 (d) 21 The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 The number of. Atomic Number Of An Element X Is 19.
From www.vecteezy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Atomic Number Of An Element X Is 19 Determine the electronic configuration of element 'x' with atomic number 19. The atomic number of an element x is 19. The list is ordered by increasing atomic number, which is the number of. If the number of protons in one atom of an element y is 20, then the number of electrons in its ion y 2 + will be:. Atomic Number Of An Element X Is 19.
From www.alamy.com
Black and white vector graphic of the symbol of the Sulphur (S) element Atomic Number Of An Element X Is 19 Determine the electronic configuration of element 'x' with atomic number 19. The atomic number of an element x is 19. The number of electrons in its ion x + will be: (a) 20 (b) 19 (c) 18 (d) 16 the element. The atomic number of an element x is 19. (a) 18 (b) 19 (c) 20 (d) 21 50% students. Atomic Number Of An Element X Is 19.
From www.doubtnut.com
The atomic number of an element X is 16 (a) Write down the electric Atomic Number Of An Element X Is 19 The list is ordered by increasing atomic number, which is the number of. B) it belongs to fourth period and its valency is 1. 119 rows here is a list of elements of the periodic table, their atomic numbers, and element symbols. The atomic number of an element x is 19. The atomic number of an element x is 19.. Atomic Number Of An Element X Is 19.