Which Has Higher Surface Tension Water Or Mercury . Next to mercury, water has the highest surface tension of all commonly occurring liquids. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is a manifestation of the presence of. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Lakes of missouri) within a body of a liquid, a molecule will not experience a. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. In fact, other than mercury, water has the greatest surface tension of any liquid. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus.
from mavink.com
Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of the presence of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Surface tension is the energy required to increase the surface area of a liquid by a given amount. In fact, other than mercury, water has the greatest surface tension of any liquid. Equivalently, it can be stated as surface energy in ergs per square centimeter. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,.
Water Molecules Surface Tension
Which Has Higher Surface Tension Water Or Mercury Surface tension is a manifestation of the presence of. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is a manifestation of the presence of. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. In fact, other than mercury, water has the greatest surface tension of any liquid. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Equivalently, it can be stated as surface energy in ergs per square centimeter. Lakes of missouri) within a body of a liquid, a molecule will not experience a. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Next to mercury, water has the highest surface tension of all commonly occurring liquids.
From philschatz.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action · Physics Which Has Higher Surface Tension Water Or Mercury What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is a manifestation of the presence of. Water has a surface tension of 0.07275 joule per square metre at 20. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
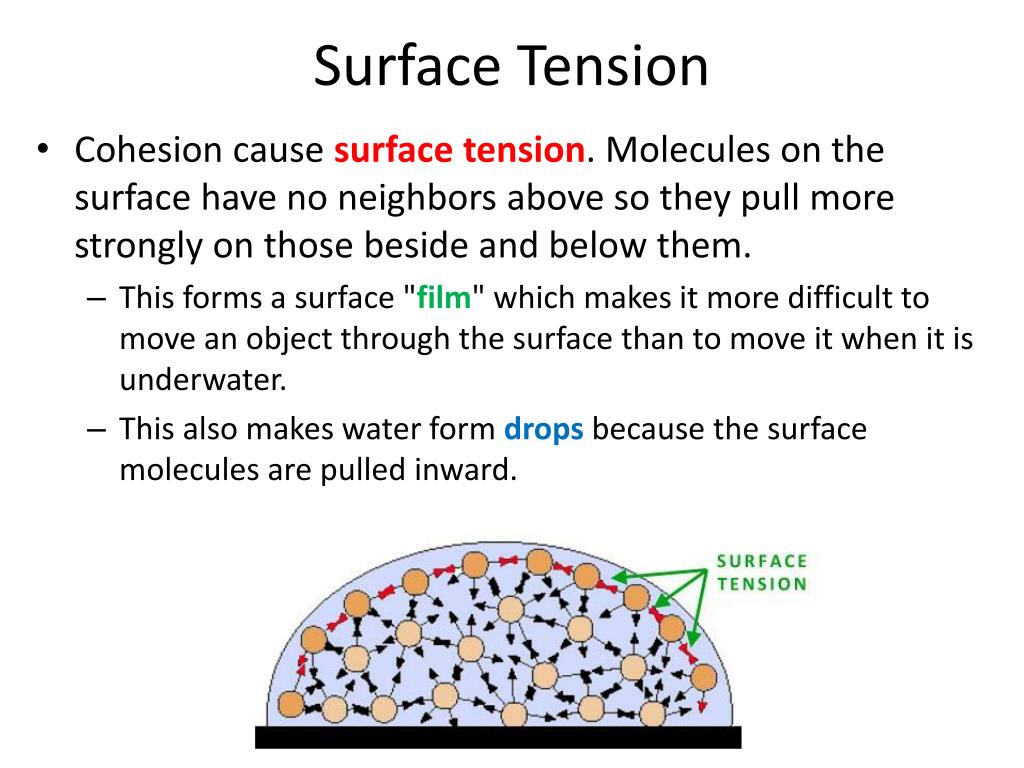
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Which Has Higher Surface Tension Water Or Mercury (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Surface tension is the energy required to increase the surface area of a liquid by a given amount. What i knew and what i found relevant is the high surface tension. Which Has Higher Surface Tension Water Or Mercury.
From hxedgviga.blob.core.windows.net
What Causes High Surface Tension Of Water at Todd James blog Which Has Higher Surface Tension Water Or Mercury Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is a manifestation of the presence of. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Equivalently, it can be stated as surface energy in ergs per square centimeter. Lakes of missouri) within a. Which Has Higher Surface Tension Water Or Mercury.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Which Has Higher Surface Tension Water Or Mercury Lakes of missouri) within a body of a liquid, a molecule will not experience a. Equivalently, it can be stated as surface energy in ergs per square centimeter. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. In comparison, organic liquids, such as benzene and alcohols , have lower. Which Has Higher Surface Tension Water Or Mercury.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with High Surface Tension in Real Which Has Higher Surface Tension Water Or Mercury Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In fact, other than mercury, water has the greatest surface tension of any liquid. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has. Which Has Higher Surface Tension Water Or Mercury.
From www.numerade.com
At 60^∘ C the surface tension of mercury and water is 0.47 and 0.0662 N / m, respectively. What Which Has Higher Surface Tension Water Or Mercury Lakes of missouri) within a body of a liquid, a molecule will not experience a. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface. Which Has Higher Surface Tension Water Or Mercury.
From www.biolinscientific.com
Surface tension of water Why is it so high? Which Has Higher Surface Tension Water Or Mercury In fact, other than mercury, water has the greatest surface tension of any liquid. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. In comparison, organic liquids, such as. Which Has Higher Surface Tension Water Or Mercury.
From www.numerade.com
SOLVED Identify the true statements about surface tension. Molecules along the surface of a Which Has Higher Surface Tension Water Or Mercury Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. In fact, other than mercury, water has the greatest surface tension of any liquid. Lakes of missouri) within a body of a liquid, a molecule. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download ID2019622 Which Has Higher Surface Tension Water Or Mercury Lakes of missouri) within a body of a liquid, a molecule will not experience a. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is a manifestation of the presence of. Equivalently, it can be stated as surface energy in ergs per square centimeter. In comparison, organic liquids, such. Which Has Higher Surface Tension Water Or Mercury.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Which Has Higher Surface Tension Water Or Mercury (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Lakes of missouri) within a body of a liquid, a molecule will not experience a. In fact, other than mercury, water has the greatest surface tension of any liquid. In comparison,. Which Has Higher Surface Tension Water Or Mercury.
From www.intec-america.com
Mercury in Drinking Water Causes, Effects, and Prevention Explained Intec America Corporation Which Has Higher Surface Tension Water Or Mercury Surface tension is a manifestation of the presence of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. (b) because. Which Has Higher Surface Tension Water Or Mercury.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Which Has Higher Surface Tension Water Or Mercury In fact, other than mercury, water has the greatest surface tension of any liquid. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Next to mercury, water has the. Which Has Higher Surface Tension Water Or Mercury.
From mavink.com
Water Molecules Surface Tension Which Has Higher Surface Tension Water Or Mercury In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Lakes of missouri) within a body of a liquid, a molecule will not experience a. In fact, other than mercury, water has the greatest surface tension of any liquid. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID3525649 Which Has Higher Surface Tension Water Or Mercury Surface tension is a manifestation of the presence of. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Next to mercury, water has the highest surface tension of all. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID5339648 Which Has Higher Surface Tension Water Or Mercury Surface tension is a manifestation of the presence of. Equivalently, it can be stated as surface energy in ergs per square centimeter. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Next to mercury, water has the highest surface tension. Which Has Higher Surface Tension Water Or Mercury.
From fphoto.photoshelter.com
science chemistry surface tension Fundamental Photographs The Art of Science Which Has Higher Surface Tension Water Or Mercury Surface tension is a manifestation of the presence of. Lakes of missouri) within a body of a liquid, a molecule will not experience a. Surface tension is the energy required to increase the surface area of a liquid by a given amount. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. What i knew. Which Has Higher Surface Tension Water Or Mercury.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Which Has Higher Surface Tension Water Or Mercury Next to mercury, water has the highest surface tension of all commonly occurring liquids. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Lakes of missouri) within a body of a liquid, a molecule will not experience a. (b) because water adheres strongly to the polar surface of glass,. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Forces of attraction/repulsion between molecules. PowerPoint Presentation ID9701387 Which Has Higher Surface Tension Water Or Mercury Next to mercury, water has the highest surface tension of all commonly occurring liquids. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Equivalently, it can be stated as surface energy in ergs per square centimeter. In fact, other than mercury, water has the greatest surface tension of any. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 Which Has Higher Surface Tension Water Or Mercury (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. In fact, other than mercury, water has the greatest surface tension of any liquid. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. What i. Which Has Higher Surface Tension Water Or Mercury.
From www.solutionspile.com
[Solved] Let's look at the surface tension of the liqui Which Has Higher Surface Tension Water Or Mercury Lakes of missouri) within a body of a liquid, a molecule will not experience a. Next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is a manifestation of the presence of. What i knew and what i found. Which Has Higher Surface Tension Water Or Mercury.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Which Has Higher Surface Tension Water Or Mercury What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. In comparison, organic liquids, such as benzene and alcohols , have. Which Has Higher Surface Tension Water Or Mercury.
From mercury-drugs.blogspot.com
Mercury Surface Tension Mercury Drugs Which Has Higher Surface Tension Water Or Mercury Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Next to mercury, water has the highest surface tension of all commonly occurring. Which Has Higher Surface Tension Water Or Mercury.
From www.chegg.com
Solved Let's look at the surface tension of the liquid metal Which Has Higher Surface Tension Water Or Mercury Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass,. Which Has Higher Surface Tension Water Or Mercury.
From fphoto.photoshelter.com
science chemistry surface tension Fundamental Photographs The Art of Science Which Has Higher Surface Tension Water Or Mercury Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In fact, other than mercury, water has the greatest surface tension of any liquid. Equivalently, it can be stated as surface energy in ergs per square centimeter. (b) because water adheres strongly to the polar surface of glass, it has. Which Has Higher Surface Tension Water Or Mercury.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet Which Has Higher Surface Tension Water Or Mercury What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Equivalently, it can be stated as surface energy in ergs per. Which Has Higher Surface Tension Water Or Mercury.
From byjus.com
Explain why the angle of contact of mercury with glass is obtuse, while that of water with glass Which Has Higher Surface Tension Water Or Mercury Lakes of missouri) within a body of a liquid, a molecule will not experience a. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In fact, other than mercury,. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Chapter 15 “Water and Aqueous Systems” PowerPoint Presentation ID2915263 Which Has Higher Surface Tension Water Or Mercury In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation of the presence of. (b) because water adheres strongly to. Which Has Higher Surface Tension Water Or Mercury.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog Which Has Higher Surface Tension Water Or Mercury In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is a manifestation of the presence of. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus,. Which Has Higher Surface Tension Water Or Mercury.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces between water molecules Which Has Higher Surface Tension Water Or Mercury Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Lakes of missouri) within a body of a liquid, a molecule will not experience a. What i knew and what i found relevant is the high. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download ID6877497 Which Has Higher Surface Tension Water Or Mercury Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download ID314146 Which Has Higher Surface Tension Water Or Mercury Equivalently, it can be stated as surface energy in ergs per square centimeter. In fact, other than mercury, water has the greatest surface tension of any liquid. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a manifestation of the presence of. What i knew and what i found relevant is the. Which Has Higher Surface Tension Water Or Mercury.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Which Has Higher Surface Tension Water Or Mercury Surface tension is the energy required to increase the surface area of a liquid by a given amount. In fact, other than mercury, water has the greatest surface tension of any liquid. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 Which Has Higher Surface Tension Water Or Mercury In fact, other than mercury, water has the greatest surface tension of any liquid. (b) because water adheres strongly to the polar surface of glass, it has a concave meniscus, whereas mercury, which does not adhere to the glass, has a convex meniscus. Lakes of missouri) within a body of a liquid, a molecule will not experience a. What i. Which Has Higher Surface Tension Water Or Mercury.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation, free download ID565121 Which Has Higher Surface Tension Water Or Mercury In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. What i knew and what i found relevant is the high surface tension of mercury is due to intermetallic bonding. Lakes of missouri) within a body of a liquid, a molecule will not experience a. Surface tension is the energy required to increase the surface. Which Has Higher Surface Tension Water Or Mercury.
From slideplayer.com
Water, Water Everywhere! ppt download Which Has Higher Surface Tension Water Or Mercury Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy required to increase the surface area of a liquid by a given amount. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. (b) because water adheres strongly to the polar surface of glass,. Which Has Higher Surface Tension Water Or Mercury.