From www.doubtnut.com
In the galvanic cell shown below, which arrow indicates the spontaneou In A Galvanic Cell Electrons Transfer From In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. In A Galvanic Cell Electrons Transfer From.
From www.numerade.com
SOLVED Question 5 2 pts In a galvanic cell, electrons transfer from [ Select ] to [Select In A Galvanic Cell Electrons Transfer From In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. In A Galvanic Cell Electrons Transfer From.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper. In A Galvanic Cell Electrons Transfer From.
From studylib.net
Basic Physics of Galvanic Cells (1) In A Galvanic Cell Electrons Transfer From In writing the equations, it is. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through an external circuit to the copper electrode. Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download ID2281331 In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. Here the. In A Galvanic Cell Electrons Transfer From.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through an external circuit to the copper electrode. In writing the equations, it is. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a. In A Galvanic Cell Electrons Transfer From.
From glossary.periodni.com
Bunsenov članak Chemistry Dictionary & Glossary In A Galvanic Cell Electrons Transfer From In writing the equations, it is. Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download ID2281530 In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 In A Galvanic Cell Electrons Transfer From Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge In A Galvanic Cell Electrons Transfer From Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From askfilo.com
ELECTROCHEMICAL CELLS AND GALVAN CELL 1. In a galvanic cell, electron flo.. In A Galvanic Cell Electrons Transfer From The electrons travel through through an external circuit to the copper electrode. Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical. In A Galvanic Cell Electrons Transfer From.
From www.bigstockphoto.com
Galvanic Cell Simple & Easy Image & Photo Bigstock In A Galvanic Cell Electrons Transfer From Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a. In A Galvanic Cell Electrons Transfer From.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical. In A Galvanic Cell Electrons Transfer From.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. In A Galvanic Cell Electrons Transfer From.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle In A Galvanic Cell Electrons Transfer From Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Here the cu²⁺(aq) ions in contact with the cu. In writing the equations, it is. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From studylib.net
Galvanic Cell Concept In A Galvanic Cell Electrons Transfer From In writing the equations, it is. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From 2012books.lardbucket.org
Describing Electrochemical Cells In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a. In A Galvanic Cell Electrons Transfer From.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE In A Galvanic Cell Electrons Transfer From The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. In A Galvanic Cell Electrons Transfer From.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and reduction.Simple In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Galvanic Cells Converting chemical energy to electrical energy PowerPoint Presentation In A Galvanic Cell Electrons Transfer From The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. Here the cu²⁺(aq) ions in contact with the. In A Galvanic Cell Electrons Transfer From.
From saylordotorg.github.io
Electrochemistry In A Galvanic Cell Electrons Transfer From In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through an external circuit to the copper electrode. Here the. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download ID2281331 In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper electrode. Here the. In A Galvanic Cell Electrons Transfer From.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Here the. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Presentation ID9388031 In A Galvanic Cell Electrons Transfer From The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In writing the equations, it is. Here the. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5404890 In A Galvanic Cell Electrons Transfer From In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a. In A Galvanic Cell Electrons Transfer From.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From galvanizeit.org
Galvanic Corrosion & Galvanic Cell… American Galvanizers Association In A Galvanic Cell Electrons Transfer From Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Here the. In A Galvanic Cell Electrons Transfer From.
From courses.lumenlearning.com
Galvanic Cells Chemistry In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through. In A Galvanic Cell Electrons Transfer From.
From www.doubtnut.com
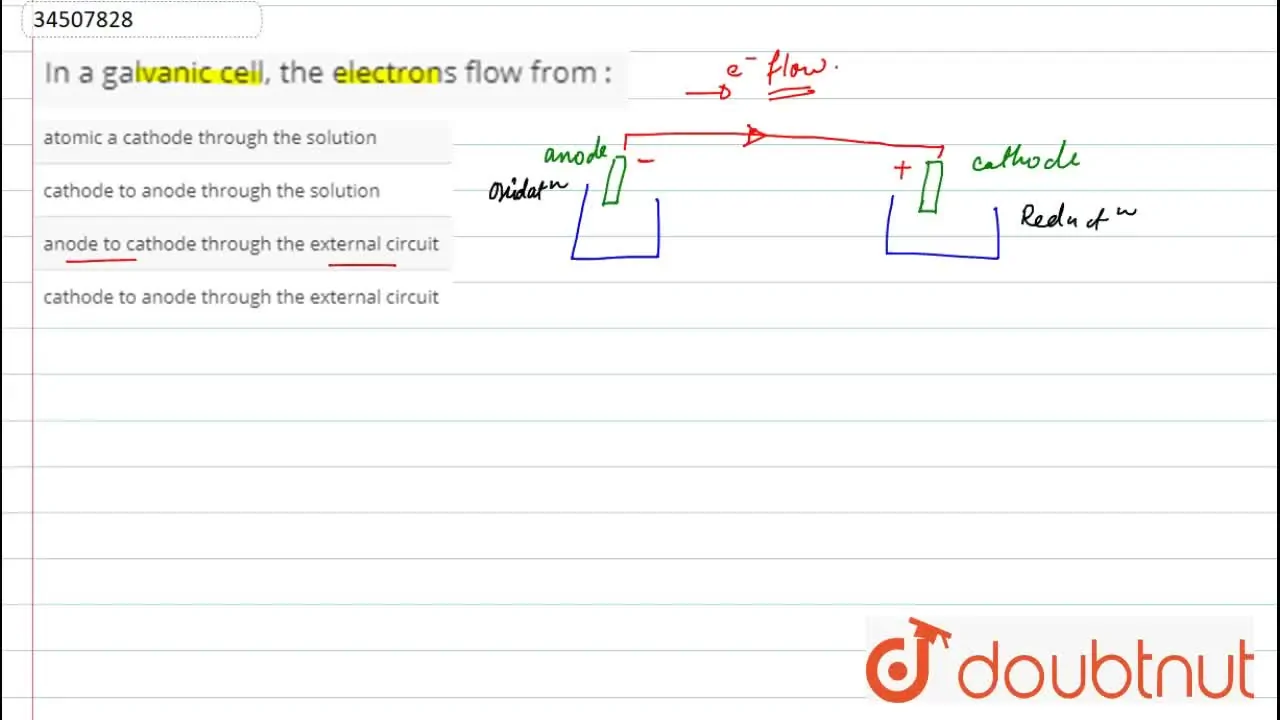
In a galvanic cell, the electrons flow from In A Galvanic Cell Electrons Transfer From In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Here the. In A Galvanic Cell Electrons Transfer From.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download ID2281530 In A Galvanic Cell Electrons Transfer From In writing the equations, it is. The electrons travel through through an external circuit to the copper electrode. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Here the. In A Galvanic Cell Electrons Transfer From.
From courses.lumenlearning.com
Galvanic Cells Chemistry In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical. In A Galvanic Cell Electrons Transfer From.
From biochemreview.weebly.com
Galvanic cell BIOChemReview In A Galvanic Cell Electrons Transfer From Here the cu²⁺(aq) ions in contact with the cu. The electrons travel through through an external circuit to the copper electrode. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical. In A Galvanic Cell Electrons Transfer From.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Electrochemistry YouTube In A Galvanic Cell Electrons Transfer From In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. The electrons travel through through an external circuit to the copper electrode. In writing the equations, it is. Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. Here the. In A Galvanic Cell Electrons Transfer From.