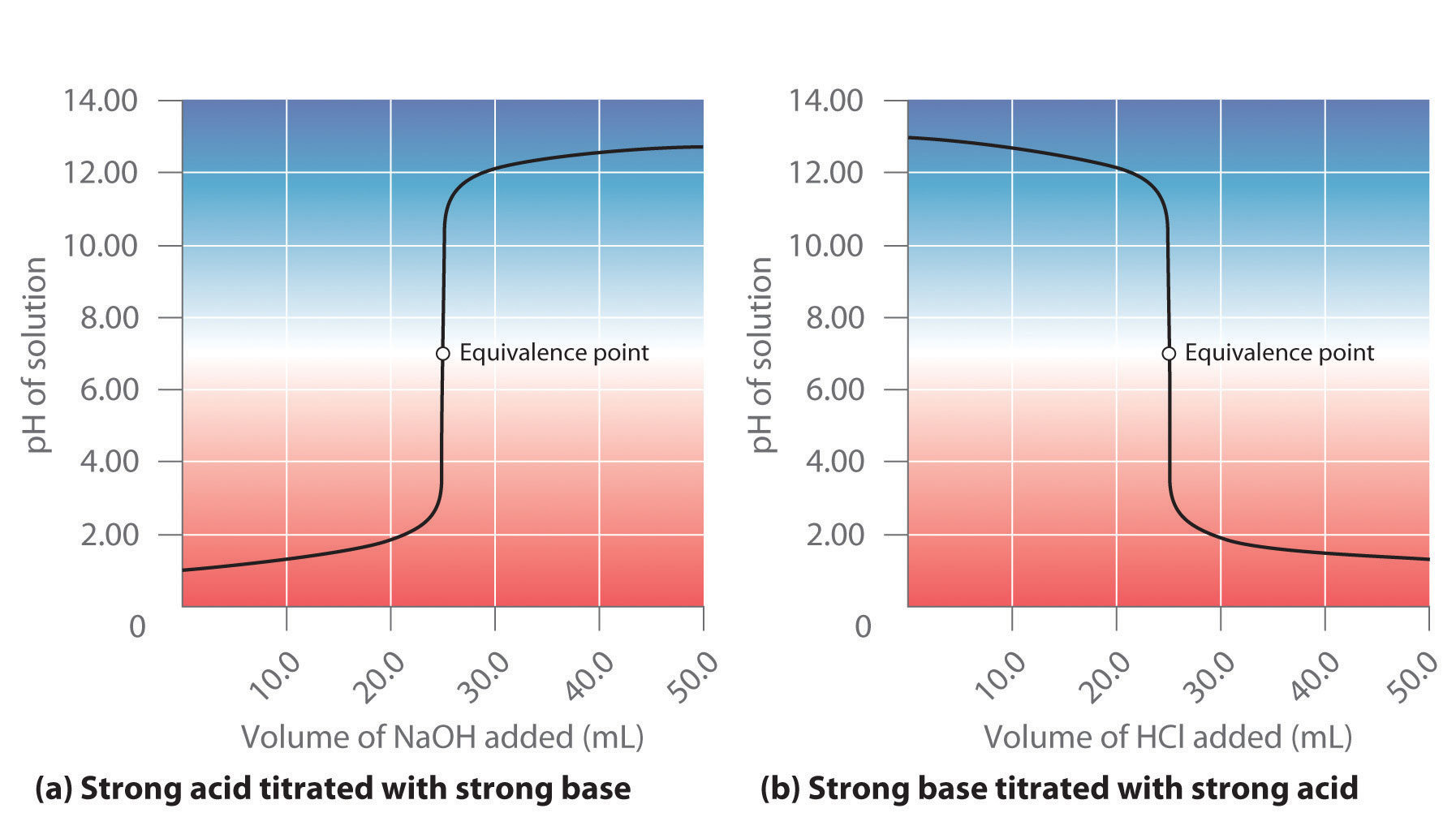
End Point Of Titration Acid . The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid and a base), there are two common ways to find the endpoint:
from saylordotorg.github.io
For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator.
AcidBase Titrations
End Point Of Titration Acid Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator.
From studylib.net
Titration Curve weak base with strong acid START End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the indicator tells us to end the titration, to stop. End Point Of Titration Acid.
From www.animalia-life.club
Endpoint Titration End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the. End Point Of Titration Acid.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 End Point Of Titration Acid Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the. End Point Of Titration Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps End Point Of Titration Acid Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution. End Point Of Titration Acid.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point Of Titration Acid Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution. End Point Of Titration Acid.
From vdocuments.mx
Determining the End Point in the Titration of Sulphuric Acid and Sodium End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. The colour change of the. End Point Of Titration Acid.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. The colour change of the. End Point Of Titration Acid.
From philschatz.com
AcidBase Titrations · Chemistry End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of. End Point Of Titration Acid.
From dokumen.tips
(PPT) TITRATION Titration of a strong acid with a strong base ENDPOINT End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed. End Point Of Titration Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed. End Point Of Titration Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Acidity is determined by titrating with. End Point Of Titration Acid.
From www.slideserve.com
PPT Buffers and Acid/Base Titration PowerPoint Presentation, free End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the. End Point Of Titration Acid.
From www.youtube.com
AcidBase Titration (Thymolphthalein end point) YouTube End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of. End Point Of Titration Acid.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle End Point Of Titration Acid Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed. End Point Of Titration Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with. End Point Of Titration Acid.
From www.sliderbase.com
Titration End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid. End Point Of Titration Acid.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. Acidity is determined by titrating with. End Point Of Titration Acid.
From www.animalia-life.club
Endpoint Titration End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. The colour change of the. End Point Of Titration Acid.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with. End Point Of Titration Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with. End Point Of Titration Acid.
From saylordotorg.github.io
AcidBase Titrations End Point Of Titration Acid Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution. End Point Of Titration Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a. End Point Of Titration Acid.
From www.microlit.com
An Advanced Guide to Titration Microlit End Point Of Titration Acid Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed. End Point Of Titration Acid.
From schoolbag.info
If the acetic acid being titrated here were replaced by hydrochloric End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. The colour change of the. End Point Of Titration Acid.
From www.youtube.com
AcidBase Titration (Methyl orange end point) YouTube End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the indicator tells us to end the titration, to stop. End Point Of Titration Acid.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube End Point Of Titration Acid Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the. End Point Of Titration Acid.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the. End Point Of Titration Acid.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of. End Point Of Titration Acid.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators End Point Of Titration Acid Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed. End Point Of Titration Acid.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry End Point Of Titration Acid For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the. End Point Of Titration Acid.
From www.vecteezy.com
Acid base titration experiment and phases of color change during End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid. End Point Of Titration Acid.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid. End Point Of Titration Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry End Point Of Titration Acid Acidity is determined by titrating with a standard solution of naoh to fixed ph of 3.7 (or the bromothymol blue end point) and a fixed ph 8.3. Using a ph probe and using a colour indicator. The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution. End Point Of Titration Acid.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Acidity is determined by titrating with. End Point Of Titration Acid.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki End Point Of Titration Acid The colour change of the indicator tells us to end the titration, to stop adding any more base to the acid because the solution has been. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Acidity is determined by titrating with. End Point Of Titration Acid.