Air In Cylinder Is Suddenly Compressed By A Piston . The gas is suddenly compressed by pressing piston downwards and is maintained at this. Due to compression the temperature of the system increases to a. as temperature decreases with time, pressure decreases too. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. The pressure of the gas on the cylinder will try to balance the weight of the piston. a gas is contained in a metallic cylinder fitted with a pistons. Air in a cylinder is suddenly compressed by a piston, which. The correct option is a the pressure decreases. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position.
from www.numerade.com
an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The pressure of the gas on the cylinder will try to balance the weight of the piston. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. Due to compression the temperature of the system increases to a. Air in a cylinder is suddenly compressed by a piston, which. The correct option is a the pressure decreases. as temperature decreases with time, pressure decreases too. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. a gas is contained in a metallic cylinder fitted with a pistons. The gas is suddenly compressed by pressing piston downwards and is maintained at this.
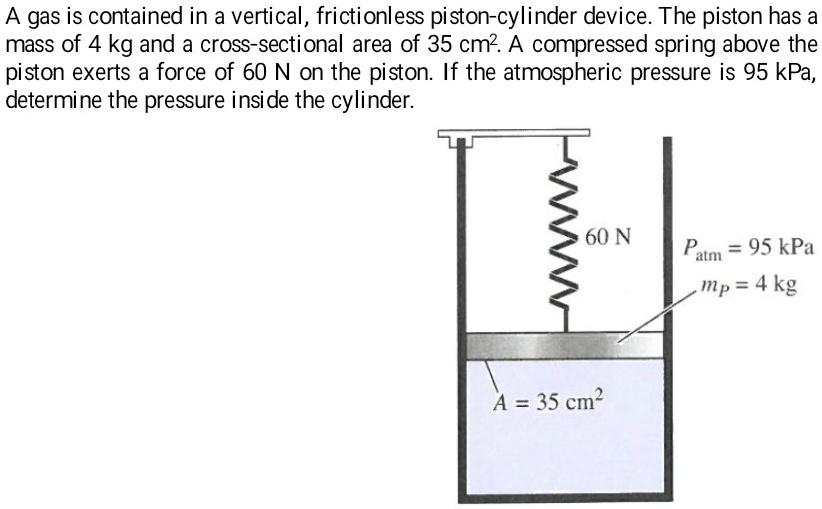
A gas is contained in a vertical, frictionless pistoncylinder device
Air In Cylinder Is Suddenly Compressed By A Piston a gas is contained in a metallic cylinder fitted with a pistons. a gas is contained in a metallic cylinder fitted with a pistons. Due to compression the temperature of the system increases to a. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The gas is suddenly compressed by pressing piston downwards and is maintained at this. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. The correct option is a the pressure decreases. as temperature decreases with time, pressure decreases too. Air in a cylinder is suddenly compressed by a piston, which. The pressure of the gas on the cylinder will try to balance the weight of the piston. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the.
From www.researchgate.net
(PDF) Investigation of friction properties in the pistoncylinder liner Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The pressure of the gas on the cylinder will try to balance the weight of the piston. as temperature decreases with time, pressure decreases too. Due to compression the temperature. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.toppr.com
Air in a cylinder is suddenly compressed by a piston which is then Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. a gas is contained in a metallic cylinder fitted with a pistons. The pressure of the gas on the cylinder will try to balance the weight of the piston. Air in a cylinder is suddenly compressed by a piston, which. The gas is suddenly compressed by pressing piston downwards and is. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.britannica.com
Piston and cylinder Engineering, Mechanics & Applications Britannica Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. a gas is contained in a metallic cylinder fitted with a pistons. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.youtube.com
Pneumatic Cylinder Working explained (Animation) YouTube Air In Cylinder Is Suddenly Compressed By A Piston The gas is suddenly compressed by pressing piston downwards and is maintained at this. The pressure of the gas on the cylinder will try to balance the weight of the piston. Due to compression the temperature of the system increases to a. Air in a cylinder is suddenly compressed by a piston, which. The correct option is a the pressure. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved A pistoncylinder device contains air initially at Air In Cylinder Is Suddenly Compressed By A Piston as temperature decreases with time, pressure decreases too. Due to compression the temperature of the system increases to a. a gas is contained in a metallic cylinder fitted with a pistons. The correct option is a the pressure decreases. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.solutionspile.com
[Solved] A pistoncylinder arrangement contains air at Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. as temperature decreases with time, pressure decreases too. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. a gas is contained in a metallic cylinder fitted with a pistons. an ideal monoatomic gas is confined in a cylinder. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Air contained within a pistoncylinder assembly is Air In Cylinder Is Suddenly Compressed By A Piston Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. The correct option is a the pressure decreases. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. The pressure of the gas on the cylinder will try. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Air is compressed in a pistoncylinder device from Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. The gas is suddenly compressed by pressing piston downwards and is maintained at this. Due to compression the temperature of the system increases to a. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. since the sudden compression causes. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved An externally adiabatic piston cylinder system Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. Air in a cylinder is suddenly compressed by a piston, which. Step by step video solution for air in a cylinder is. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.youtube.com
Air in a cylinder is suddenly compressed by a piston, which is then Air In Cylinder Is Suddenly Compressed By A Piston The gas is suddenly compressed by pressing piston downwards and is maintained at this. a gas is contained in a metallic cylinder fitted with a pistons. Air in a cylinder is suddenly compressed by a piston, which. The correct option is a the pressure decreases. Step by step video solution for air in a cylinder is suddenly compressed by. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved 1. As Shown In Fig Below, A Pistoncylinder Assemb... Air In Cylinder Is Suddenly Compressed By A Piston Air in a cylinder is suddenly compressed by a piston, which. as temperature decreases with time, pressure decreases too. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.youtube.com
A pistoncylinder device contains 0.15 kg of air initially at 2 MPa and Air In Cylinder Is Suddenly Compressed By A Piston The gas is suddenly compressed by pressing piston downwards and is maintained at this. a gas is contained in a metallic cylinder fitted with a pistons. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. Air in a cylinder is suddenly compressed by a piston, which. . Air In Cylinder Is Suddenly Compressed By A Piston.
From brainly.com
Air is compressed slowly in a pistoncylinder assembly from an initial Air In Cylinder Is Suddenly Compressed By A Piston an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The gas is suddenly compressed by pressing piston downwards and is maintained at this. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. as temperature. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.numerade.com
A gas is contained in a vertical, frictionless pistoncylinder device Air In Cylinder Is Suddenly Compressed By A Piston The gas is suddenly compressed by pressing piston downwards and is maintained at this. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. as temperature decreases with time, pressure decreases too. Step by step video solution for air in a cylinder is suddenly compressed by a piston,. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved 472 A mass of 15 kg of air in a pistoncylinder Air In Cylinder Is Suddenly Compressed By A Piston Due to compression the temperature of the system increases to a. as temperature decreases with time, pressure decreases too. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. a gas is contained in a metallic cylinder fitted with a pistons. The pressure of the gas on. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.doubtnut.com
[Punjabi] Air in a cylinder is suddenly compressed by a piston, which Air In Cylinder Is Suddenly Compressed By A Piston Due to compression the temperature of the system increases to a. The gas is suddenly compressed by pressing piston downwards and is maintained at this. The pressure of the gas on the cylinder will try to balance the weight of the piston. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Air, 5.37kg, is compressed in a pistoncylinder Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. a gas is contained in a metallic cylinder fitted with a pistons. The correct option is a the pressure decreases. as temperature decreases with time, pressure decreases too. an ideal monoatomic gas is confined in a cylinder by a spring. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.sarthaks.com
A monoatomic gas is enclosed in a nonconducting cylinder having a Air In Cylinder Is Suddenly Compressed By A Piston Air in a cylinder is suddenly compressed by a piston, which. a gas is contained in a metallic cylinder fitted with a pistons. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. Due to compression the temperature of the system increases to a. The gas is. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.youtube.com
How does a pneumatic cylinder work? Pneumatic cylinder design YouTube Air In Cylinder Is Suddenly Compressed By A Piston as temperature decreases with time, pressure decreases too. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. a gas is contained in a metallic cylinder fitted with a pistons. Air in a cylinder is suddenly compressed by a piston, which. The gas is suddenly compressed. Air In Cylinder Is Suddenly Compressed By A Piston.
From library.automationdirect.com
Pneumatic Actuator/Air Cylinder Basics Library.AutomationDirect Air In Cylinder Is Suddenly Compressed By A Piston Due to compression the temperature of the system increases to a. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The gas is suddenly compressed by pressing piston. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Air contained within a pistoncylinder assembly is Air In Cylinder Is Suddenly Compressed By A Piston Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. Air in a cylinder is suddenly compressed by a piston, which. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. The correct option is a the pressure. Air In Cylinder Is Suddenly Compressed By A Piston.
From advanxis.com
What is a Pneumatic Cylinder? Advanxis Technologies and Process Air In Cylinder Is Suddenly Compressed By A Piston as temperature decreases with time, pressure decreases too. Due to compression the temperature of the system increases to a. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.iqsdirectory.com
Pneumatic Cylinder What Is It? How Does It Work? Types Of Air In Cylinder Is Suddenly Compressed By A Piston since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. The correct option is a the pressure decreases. as temperature decreases with time, pressure decreases too. a gas is contained in a metallic cylinder fitted with a pistons. The pressure of the gas on the cylinder will try. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.numerade.com
SOLVED A 15000 N car on a hydraulic lift rests on a cylinder with a Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. as temperature decreases with time, pressure decreases too. Air in a cylinder is suddenly compressed by a piston, which. The. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Air is compressed in a pistoncylinder assembly from Air In Cylinder Is Suddenly Compressed By A Piston Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. as temperature decreases with time, pressure decreases too. The correct option is a the pressure decreases. . Air In Cylinder Is Suddenly Compressed By A Piston.
From www.britannica.com
Compressed air Energy Efficiency, Industrial Uses & Safety Britannica Air In Cylinder Is Suddenly Compressed By A Piston Air in a cylinder is suddenly compressed by a piston, which. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. The correct option is a the pressure decreases. Due to compression the temperature of the system increases to a. as temperature decreases with time, pressure decreases. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.junchumaterial.com
Aluminum Compressed Air Cylinder Compact Pneumatic Piston Cylinder Tube Air In Cylinder Is Suddenly Compressed By A Piston Due to compression the temperature of the system increases to a. The pressure of the gas on the cylinder will try to balance the weight of the piston. as temperature decreases with time, pressure decreases too. Air in a cylinder is suddenly compressed by a piston, which. an ideal monoatomic gas is confined in a cylinder by a. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.youtube.com
Pneumatic Cylinder Double Acting Air Piston YouTube Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. Air in a cylinder is suddenly compressed by a piston, which. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. since the sudden compression causes heating and rise in temperature. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved A pistoncylinder assembly contains air as shown Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. as temperature decreases with time, pressure decreases too. Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. an ideal monoatomic gas is confined in a cylinder by a. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved A frictionless pistoncylinder device contains air Air In Cylinder Is Suddenly Compressed By A Piston Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. The pressure of the gas on the cylinder will try to balance the weight of the piston. Due to compression the temperature of the system increases to a. Air in a cylinder is suddenly compressed by a piston,. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.nellisauction.com
Baomain Pneumatic Air Cylinder SC 100 x 350 PT1/2; Bore 4", Stroke 14 Air In Cylinder Is Suddenly Compressed By A Piston an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The pressure of the gas on the cylinder will try to balance the weight of the piston. The correct option is a the pressure decreases. Due to compression the temperature of the system increases to a. The gas is. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.chegg.com
Solved Q4. A compressed air cylinder is connected to the Air In Cylinder Is Suddenly Compressed By A Piston The gas is suddenly compressed by pressing piston downwards and is maintained at this. Air in a cylinder is suddenly compressed by a piston, which. as temperature decreases with time, pressure decreases too. Due to compression the temperature of the system increases to a. The pressure of the gas on the cylinder will try to balance the weight of. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.toppr.com
A gas is contained in a metallic cylinder fitted with a piston. The gas Air In Cylinder Is Suddenly Compressed By A Piston Step by step video solution for air in a cylinder is suddenly compressed by a piston, which is then maintained at the. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position. Air in a cylinder is suddenly compressed by a piston, which. The gas is suddenly compressed by pressing. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.peninsularcylinders.com
Peninsular Cylinder Co. cylinder repair, hydraulic cylinder repair Air In Cylinder Is Suddenly Compressed By A Piston The correct option is a the pressure decreases. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. Due to compression the temperature of the system increases to a. since the sudden compression causes heating and rise in temperature and if the piston is maintained at same position.. Air In Cylinder Is Suddenly Compressed By A Piston.
From www.toppr.com
Air in a cylinder is suddenly compressed by a piston, which is then Air In Cylinder Is Suddenly Compressed By A Piston The pressure of the gas on the cylinder will try to balance the weight of the piston. The gas is suddenly compressed by pressing piston downwards and is maintained at this. an ideal monoatomic gas is confined in a cylinder by a spring loaded piston of cross section 8.0×10 −3m 2. The correct option is a the pressure decreases.. Air In Cylinder Is Suddenly Compressed By A Piston.