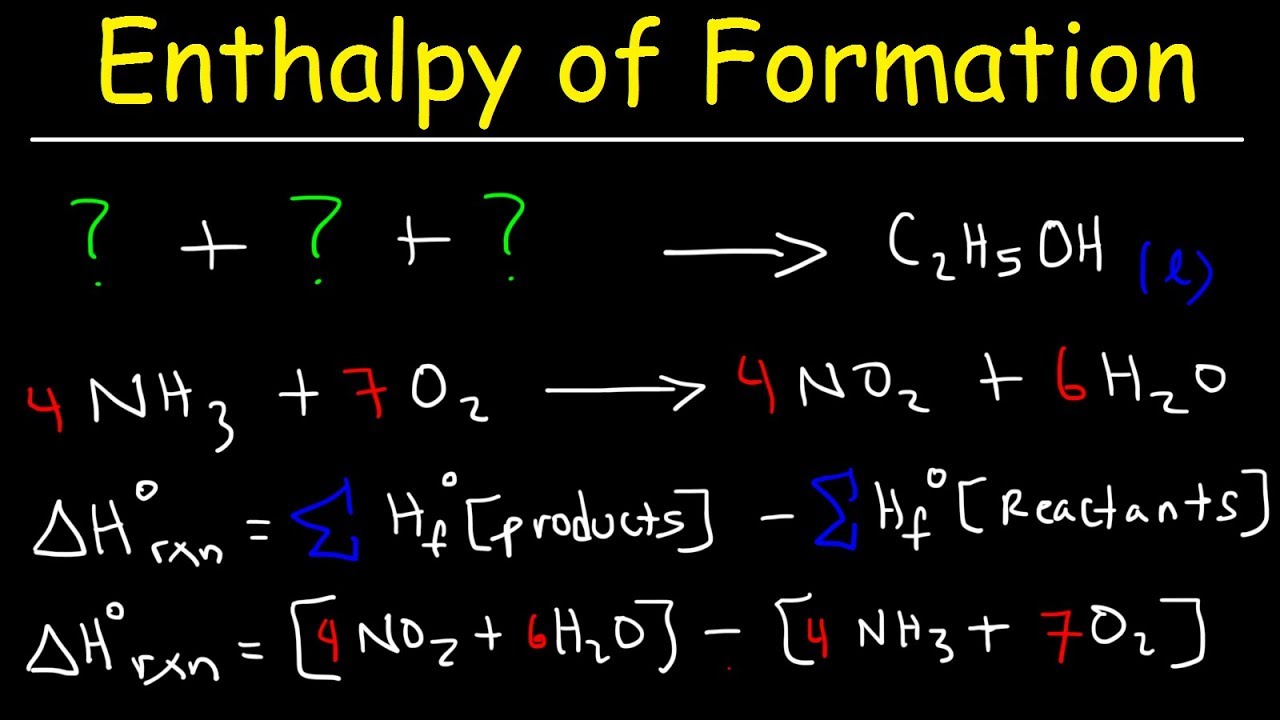
Can You Calculate Standard Enthalpy Change Of Formation . a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. this chemistry video tutorial focuses on the calculation of the. explore the foundations of thermochemistry with our standard formation enthalpy calculator! this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and.
from mungfali.com
this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. explore the foundations of thermochemistry with our standard formation enthalpy calculator! this chemistry video tutorial focuses on the calculation of the. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
Enthalpy Change Of Formation Equation
Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. this chemistry video tutorial focuses on the calculation of the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. explore the foundations of thermochemistry with our standard formation enthalpy calculator!
From mungfali.com
Standard Enthalpy Change Equation Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this chemistry video tutorial focuses on the calculation of the. tabulated values of standard enthalpies of formation. Can You Calculate Standard Enthalpy Change Of Formation.
From www.chegg.com
Solved Calculate the standard enthalpy change for the Can You Calculate Standard Enthalpy Change Of Formation this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Can You Calculate Standard Enthalpy Change Of Formation.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole. Can You Calculate Standard Enthalpy Change Of Formation.
From www.slideserve.com
PPT Energetics PowerPoint Presentation, free download ID3196359 Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$. Can You Calculate Standard Enthalpy Change Of Formation.
From brainly.com
Calculate reaction 7 and 8 for standard enthalpy change of formation Can You Calculate Standard Enthalpy Change Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. tabulated values of standard enthalpies of formation can be used to calculate enthalpy. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Can You Calculate Standard Enthalpy Change Of Formation.
From surfguppy.com
Calculate the enthalpy change of methane formation using Hess Law Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. tabulated. Can You Calculate Standard Enthalpy Change Of Formation.
From studylib.net
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. explore the foundations of thermochemistry with our standard formation enthalpy calculator! a standard enthalpy of formation $δh°_f$. Can You Calculate Standard Enthalpy Change Of Formation.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this chemistry video tutorial focuses on the calculation of the. 193. Can You Calculate Standard Enthalpy Change Of Formation.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. this chemistry video tutorial focuses on the calculation of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy. Can You Calculate Standard Enthalpy Change Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this page explains hess's law, and uses it to do some simple. Can You Calculate Standard Enthalpy Change Of Formation.
From learningzonegregorin2m.z4.web.core.windows.net
Heat Of Formation Formula Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is. Can You Calculate Standard Enthalpy Change Of Formation.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole. Can You Calculate Standard Enthalpy Change Of Formation.
From bnospmmdwi.blogspot.com
How To Calculate Standard Enthalpy Change How to calculate standard Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Can You Calculate Standard Enthalpy Change Of Formation.
From mungfali.com
Enthalpy Change Of Formation Equation Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. this chemistry video tutorial focuses on the calculation of the. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
Calculate Reaction Change in Enthalpy, Entropy and Free Energy YouTube Can You Calculate Standard Enthalpy Change Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. this page explains hess's law, and uses it to do some simple. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. the standard enthalpy. Can You Calculate Standard Enthalpy Change Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation Can You Calculate Standard Enthalpy Change Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. this chemistry video tutorial focuses on the calculation of the. this. Can You Calculate Standard Enthalpy Change Of Formation.
From mungfali.com
Standard Enthalpy Change Equation Can You Calculate Standard Enthalpy Change Of Formation the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. this chemistry video tutorial focuses on the calculation of the. 193. Can You Calculate Standard Enthalpy Change Of Formation.
From www.numerade.com
SOLVED The standard enthalpy change of formation of hydrogen iodide is Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. explore the foundations of thermochemistry with our standard formation enthalpy calculator! this chemistry video tutorial focuses. Can You Calculate Standard Enthalpy Change Of Formation.
From exoluenrv.blob.core.windows.net
Standard Enthalpy Of Formation Diamond at James Torres blog Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. explore the foundations of thermochemistry with our standard formation enthalpy calculator! . Can You Calculate Standard Enthalpy Change Of Formation.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Can You Calculate Standard Enthalpy Change Of Formation this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. explore the foundations of thermochemistry with our standard formation enthalpy calculator! 193 rows in. Can You Calculate Standard Enthalpy Change Of Formation.
From www.numerade.com
SOLVED Calculate the standard enthalpy change for the reaction at 25Â Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. this chemistry video. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube Can You Calculate Standard Enthalpy Change Of Formation this chemistry video tutorial focuses on the calculation of the. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. explore the foundations. Can You Calculate Standard Enthalpy Change Of Formation.
From www.chegg.com
Solved 15. Using standard enthalpies of formation, calculate Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. explore the foundations of thermochemistry with our standard formation enthalpy calculator! this chemistry video tutorial focuses on the calculation of the. this page explains hess's law, and uses it to do some simple enthalpy change calculations. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
[Example] How to Calculate Enthalpy Change of a Reaction. YouTube Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes. Can You Calculate Standard Enthalpy Change Of Formation.
From bxpomsyhky.blogspot.com
How To Calculate Standard Enthalpy Change Of Reaction Δh⊖ rxn =∑δh⊖ f Can You Calculate Standard Enthalpy Change Of Formation explore the foundations of thermochemistry with our standard formation enthalpy calculator! the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. tabulated. Can You Calculate Standard Enthalpy Change Of Formation.
From exoxhphey.blob.core.windows.net
Standard Enthalpy Of Formation Calculator at Susan blog Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. this chemistry video tutorial focuses on the calculation of the. this page explains hess's law, and uses it to. Can You Calculate Standard Enthalpy Change Of Formation.
From studymarxianism.z21.web.core.windows.net
How To Find Standard Enthalpy Can You Calculate Standard Enthalpy Change Of Formation this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released. Can You Calculate Standard Enthalpy Change Of Formation.
From srkvlvhthzubm.blogspot.com
How To Calculate Standard Enthalpy Change Of Formation For most Can You Calculate Standard Enthalpy Change Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Can You Calculate Standard Enthalpy Change Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Can You Calculate Standard Enthalpy Change Of Formation this chemistry video tutorial focuses on the calculation of the. this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a. Can You Calculate Standard Enthalpy Change Of Formation.
From mungfali.com
Standard Enthalpy Change Equation Can You Calculate Standard Enthalpy Change Of Formation this page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. tabulated values of standard enthalpies of formation can be used to calculate. Can You Calculate Standard Enthalpy Change Of Formation.
From www.numerade.com
SOLVED Calculate standard enthalpy change for the reaction below in kj Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. tabulated values of standard enthalpies of formation can be used to calculate. Can You Calculate Standard Enthalpy Change Of Formation.
From srkvlvhthzubm.blogspot.com
How To Calculate Standard Enthalpy Change Of Formation For most Can You Calculate Standard Enthalpy Change Of Formation a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. this page explains hess's law, and uses it to do some simple enthalpy change. Can You Calculate Standard Enthalpy Change Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Can You Calculate Standard Enthalpy Change Of Formation tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Can You Calculate Standard Enthalpy Change Of Formation.